Anticancer medical application of evofosfamide
A technology for efsolamide and cancer, applied in the field of efsolamide, which can solve the problem of inability to effectively target tumors for a long time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
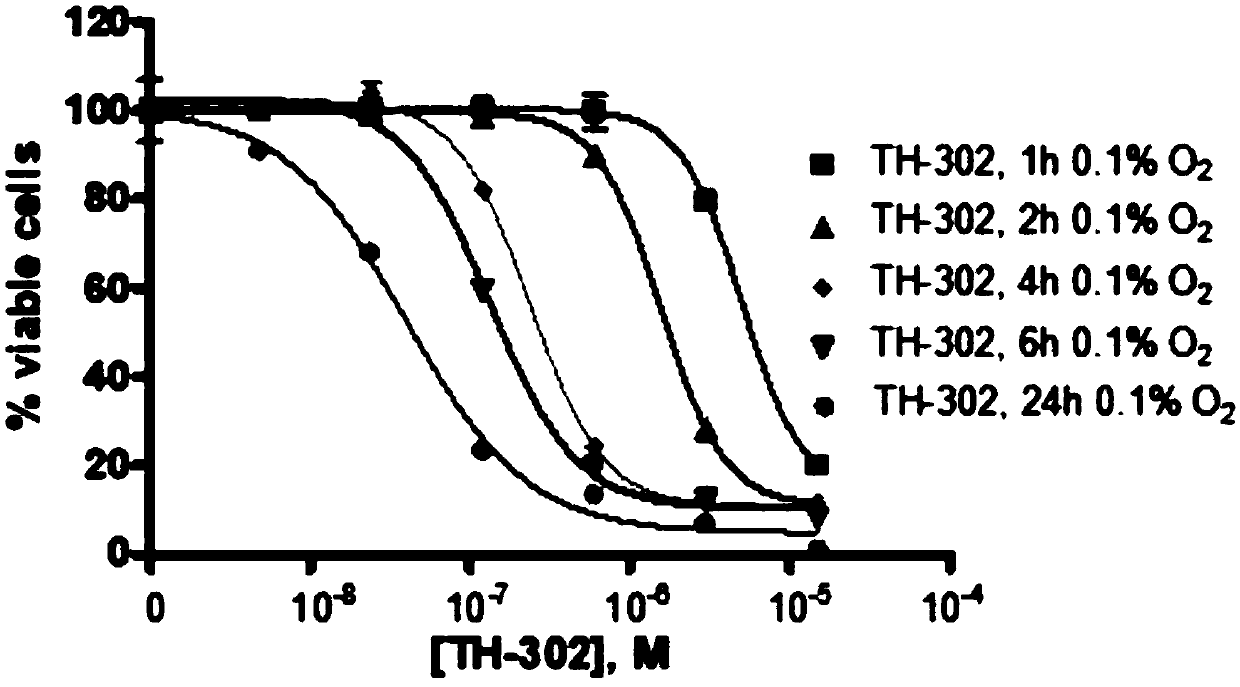
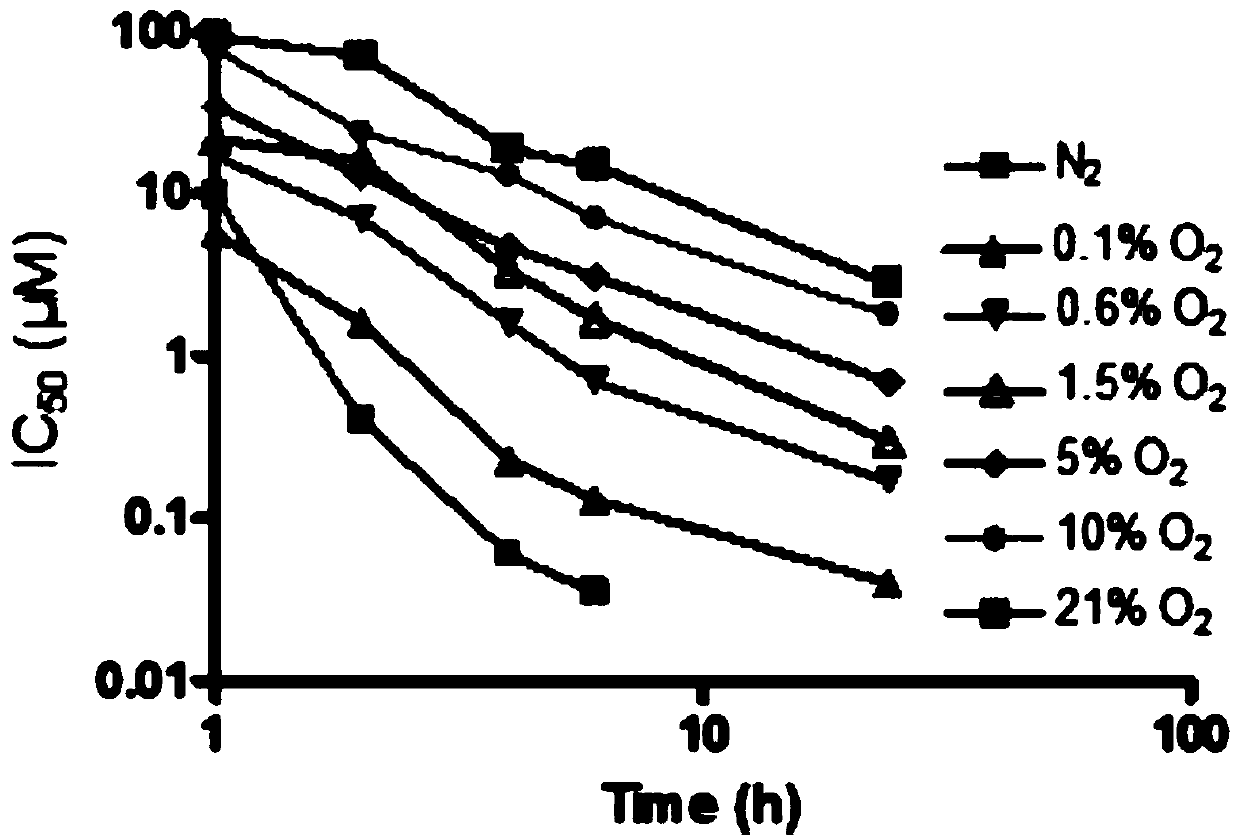
[0277] Cell Proliferation Experiment
[0278] Exponentially growing cells were seeded with different concentrations of TH-302 24 hours after inoculation. After drug addition, the plates were incubated at 37°C for 2 hours in an anaerobic chamber (Bactron II), hypoxic chamber (Hypoxystation) or standard tissue culture incubator. After washing, cells were cultured in normoxic complete medium for 72 hours. Viable cells were quantified using AlamarBlue. 50% growth inhibition (IC50) was calculated using Prism software.
[0279] In vitro clonogenic assay
[0280] Cells were seeded 24 hours before starting treatment. Cells were then incubated with drugs for 2 hours at defined oxygen concentrations. At the end of treatment, the washed cells were plated and placed in an incubator for 10 days. For VC-8, UWB1.289 and FANCA strains, cells were plated in triplicate in 6-well plastic plates with 500 to 1,000 cells per well and processed. TH-302 was cultured under hypoxic conditions fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com