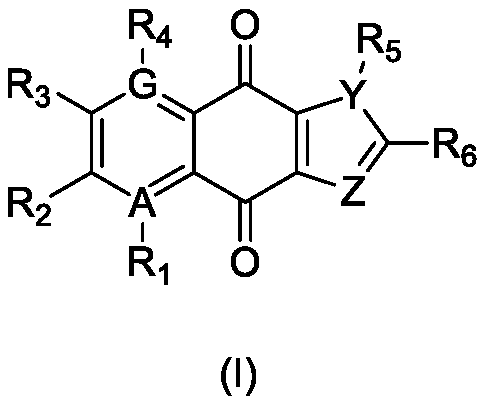
Use of naphthoquinone derivative as inhibitor for IDO1 and/or TDO
A technology of IDO1 and derivatives, which is applied in the field of application of naphthoquinone derivatives as IDO1 and/or TDO inhibitors, and can solve the problems of T cells being difficult to reactivate, unable to pass through, and depleted of the substrate tryptophan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
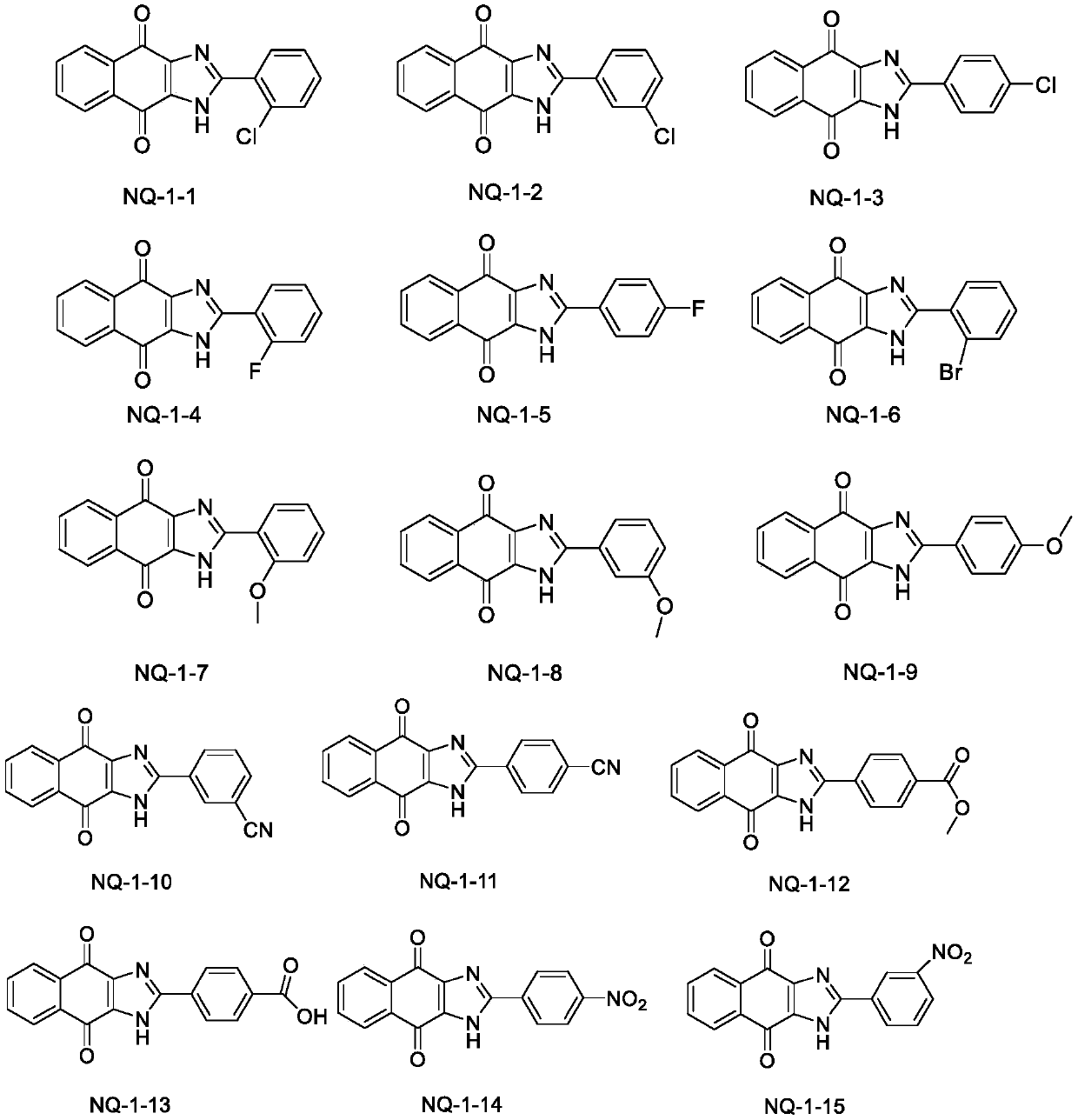
[0104] Synthesis of 2-(2-chlorophenyl)-1H-naphthalene[2,3-d]imidazole-4,9-dione (NQ-1-1)
[0105]
[0106] The compound 2,3-diamine-1,4-naphthoquinone (1mmol) was dissolved in DMF (10ml), and 2-chlorobenzaldehyde (1mmol) and sodium metabisulfite (1mmol) were added sequentially, and the reaction solution was stirred at 120°C for 8h After the completion of the reaction detected by TLC, the solvent was distilled off under reduced pressure to obtain a yellow viscous substance. After stirring and mixing the sample through silica gel, the silica gel column chromatography (dichloromethane: methanol = 60:1) separated to obtain a yellow solid. The yield was 82. %. 1 H NMR(400MHz,DMSO-d6)δ14.37(s,1H), 8.10(dd,J=6.1,3.0Hz,2H), 7.85 (dd,J=6.1,3.0Hz,2H), 7.79(d, J=7.5Hz, 1H), 7.67(d, J=7.5Hz, 1H), 7.59(t, J=7.5Hz 1H), 7.53(t, J=7.5Hz, 1H); 13 C NMR (101MHz, DMSO) δ 150.83, 134.39, 133.17, 132.65, 132.56, 132.37, 130.72, 129.09, 127.85, 126.80. HR-ESI-MS: C 17 H 9 ClN 2 O 2 Theoretical value: ...
Embodiment 2
[0108] Synthesis of 2-(3-chlorophenyl)-1H-naphthalene[2,3-d]imidazole-4,9-dione (NQ-1-2)
[0109]
[0110] Using 3-chlorobenzaldehyde instead of 2-chlorobenzaldehyde, the preparation method is the same as NQ-1-1. Brown solid, yield 86%. 1 HNMR(400MHz,DMSO-d6)δ14.52(s,1H), 8.32(dd,J=1.7Hz,1.2Hz, 1H), 8.21(ddd,J=5.0,3.5,1.7Hz,1H),8.13( dd, J = 5.7, 3.3 Hz, 2H), 7.87 (dd, J = 5.7, 3.3 Hz, 2H), 7.63-7.58 (m, 2H); 13 C NMR (101MHz, DMSO) δ151.18, 134.32, 134.24, 133.15, 131.36, 130.92, 130.65, 126.78, 126.73, 125.78. HR-ESI-MS: C 17 H 9 ClN 2 O 2 Theoretical value: 309.0425[M+H] + , Measured value: 309.0411.
Embodiment 3
[0112] Synthesis of 2-(4-chlorophenyl)-1H-naphthalene[2,3-d]o-4,9-dione (NQ-1-3)
[0113]
[0114] Use 4-chlorobenzaldehyde instead of 2-chlorobenzaldehyde, and the preparation method is the same as NQ-1-1. Yellow solid, the yield is 80%. 1 HNMR(400MHz,DMSO-d6)δ14.45(s,1H), 8.23(d,J=8.3Hz,2H), 8.09(dd,J=5.7,3.3Hz,2H),7.84(dd,J=5.8 ,3.3Hz,2H),7.62(d,J=8.4Hz, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com