Preparation method of vildagliptin
A technology of aminoadamantanol and formonitrile, which is applied in chemical instruments and methods, compounds of Group 4/14 elements of the periodic table, organic chemistry, etc., can solve problems such as reducing production efficiency and increasing production costs, and achieves improved efficiency. , improve the yield, improve the effect of crude product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (1) Preparation of 3-aminoadamantanol protected by silyl groups:
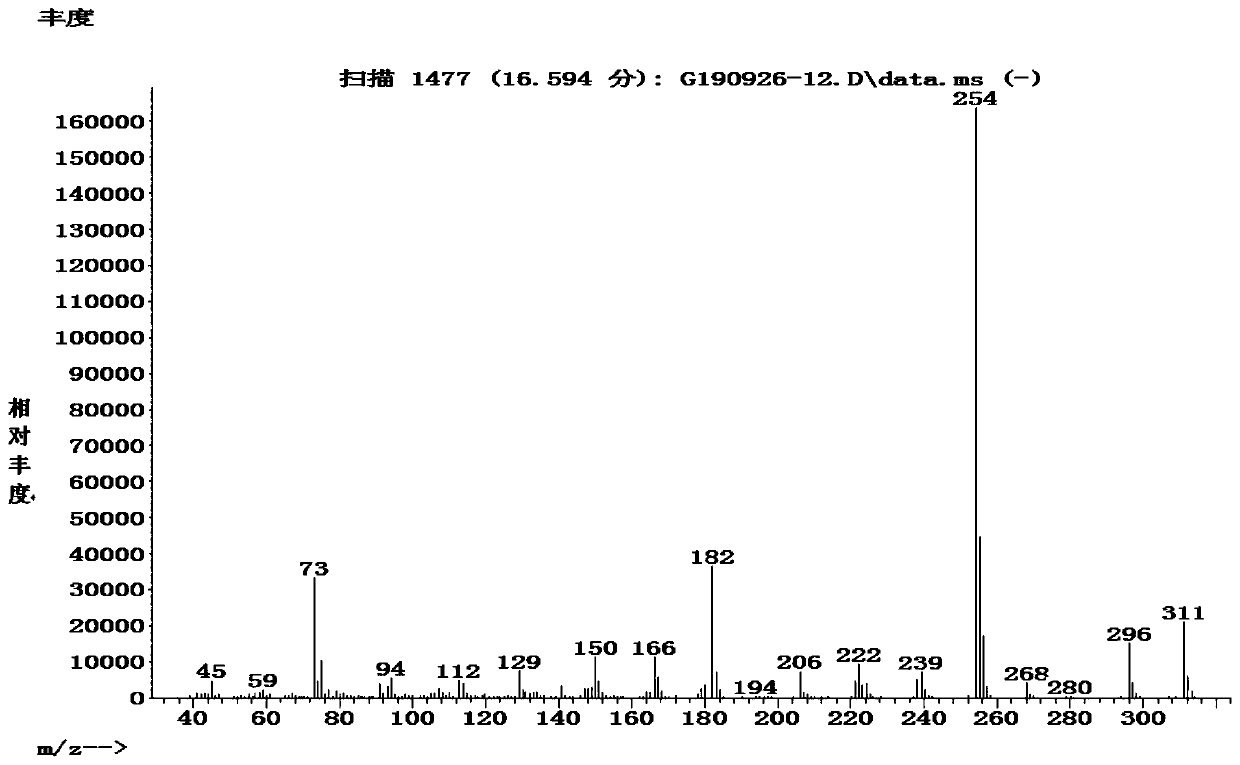
[0041] Add 117.8g (0.7299mol) 1,1,1,3,3,3-hexamethyldisilazane (HMDS for short), 116.3g (0.6954mol) 3-aminoadamantanol, 3.52 g (0.03479mol) triethylamine, 348.9g dichloromethane, heated to 35-40°C, and kept at 35-40°C for 1-6h. ) to steam off HMDS and solvent to obtain 217.0g (theoretical yield 216.7g) of light yellow liquid, yield 100.1%, GC purity 96.0%, MS spectrogram data see figure 1 , [M]=311, that is, the crude product of silyl-protected 3-aminoadamantanol.
[0042] (2) Preparation of Vildagliptin:
[0043] In a 2L three-necked flask, add 216.7g (0.6954moL) of silyl-protected 3-aminoadamantanol, 73.2g (0.7234moL) of triethylamine, 5.7g (0.03434moL) of potassium iodide and 650.0g of acetonitrile, and heat to 75 -80°C, add 118.8g (0.6883moL) (S)-1-(2-chloroacetyl chloride)pyrrolidine-2-carbonitrile (dissolved in 356.4g acetonitrile) dropwise, 2h dropwise, 75-80°C Insulated and stirred for 1 h; T...
Embodiment 2
[0045] (1) Preparation of 3-aminoadamantanol protected by silyl groups:
[0046] Add 123.5g (0.7652mol) 1,1,1,3,3,3-hexamethyldisilazane (HMDS for short), 116.3g (0.6954mol) 3-aminoadamantanol, 4.93 g (0.04872mol) triethylamine, 465.2g dichloromethane, heated to 35-40°C, and kept at 35-40°C for 1-6h. ) to distill off HMDS and solvent to obtain 217.3 g of light yellow liquid (theoretical yield 216.7 g), yield 100.3%, GC purity 97.5%, ie the crude product of silyl-protected 3-aminoadamantanol.
[0047] (2) Preparation of Vildagliptin:
[0048] In a 2L three-necked flask, add 216.7g (0.6954moL) of silyl-protected 3-aminoadamantanol, 76.3g (0.7540moL) of triethylamine, 7.6g (0.04578moL) of potassium iodide and 650.0g of acetonitrile, and heat to 75 -80°C, add dropwise 113.2g (0.6558moL) (S)-1-(2-chloroacetyl chloride)pyrrolidine-2-carbonitrile (dissolved in 339.6g acetonitrile), 2h dropwise, 75-80°C Stir under insulation for 1 h; TLC detects that the reaction is complete, add 3...
Embodiment 3
[0050] (1) Preparation of 3-aminoadamantanol protected by silyl groups:
[0051] Add 129.1g (0.7999mol) 1,1,1,3,3,3-hexamethyldisilazane (HMDS for short), 116.3g (0.6954mol) 3-aminoadamantanol, 7.04 g (0.06957mol) triethylamine, 581.5g dichloromethane, heated to 35-40°C, and kept at 35-40°C for 1-6h. ) to distill off HMDS and solvent to obtain 219.3 g of light yellow liquid (theoretical yield 216.7 g), yield 101.2%, GC purity 98.7%, ie the crude product of silyl-protected 3-aminoadamantanol.
[0052] (2) Preparation of Vildagliptin:
[0053] In a 2L three-necked flask, add 216.7g (0.6954moL) of silane-protected 3-aminoadamantanol, 114.4g (0.8852moL) of diisopropylethylamine, 10.5g (0.06325moL) of potassium iodide, 650.0g of acetonitrile, and stir Heated to 75-80°C, added dropwise 109.1g (0.6321moL) (S)-1-(2-chloroacetyl chloride)pyrrolidine-2-carbonitrile (dissolved in 327.3g acetonitrile), and the dropwise addition was completed after 2h, 75 Stir at -80°C for 1 hour; TLC d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com