A side chain type sulfonated polyquinoxaline and its proton exchange membrane
A sulfonated polyquinoxaline and sulfonated technology, applied in the field of proton exchange membranes, to achieve excellent thermal hydrolysis stability, simple and mild reaction conditions, and good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
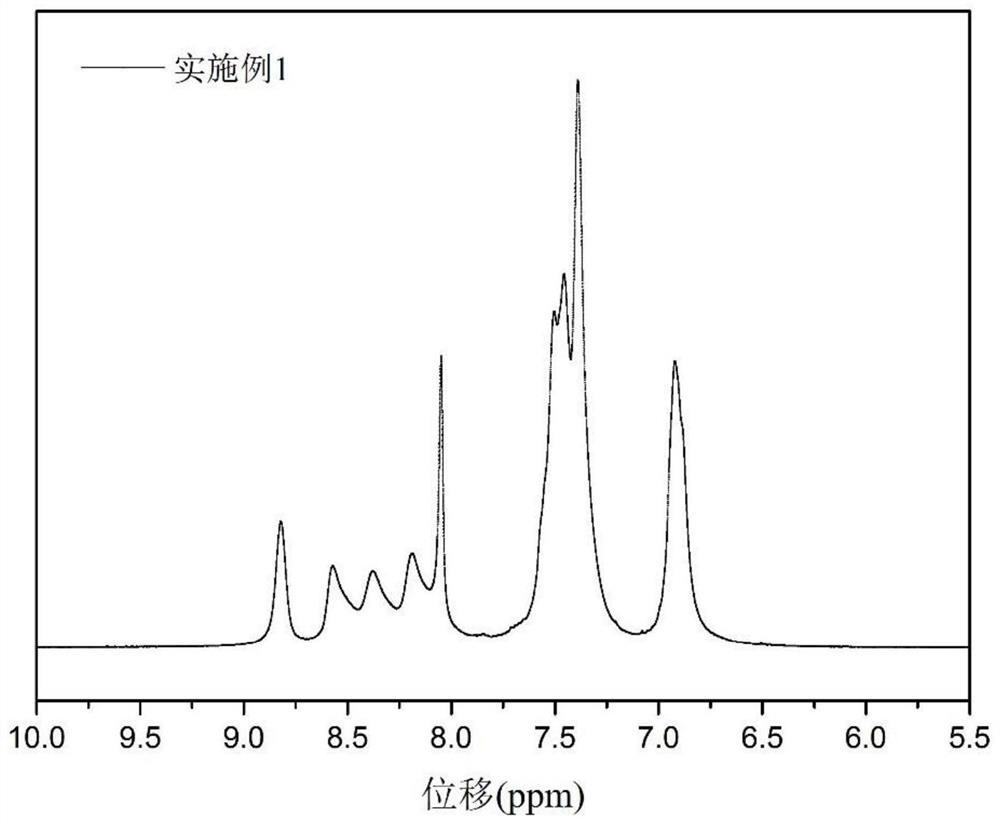
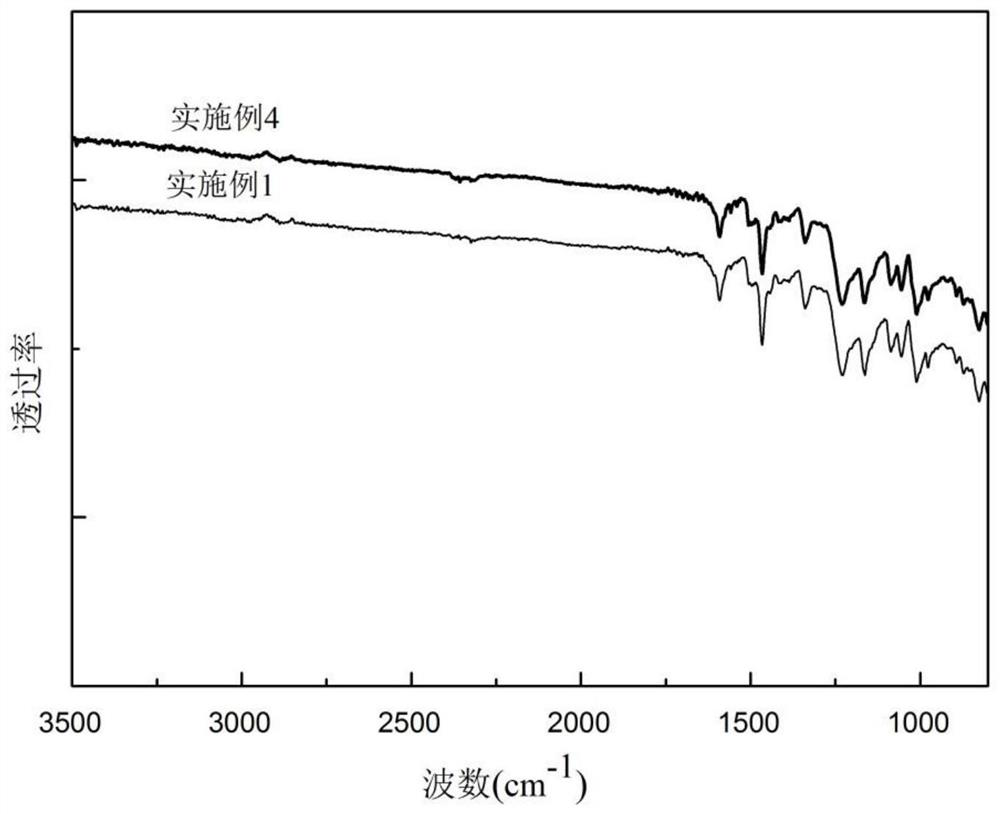
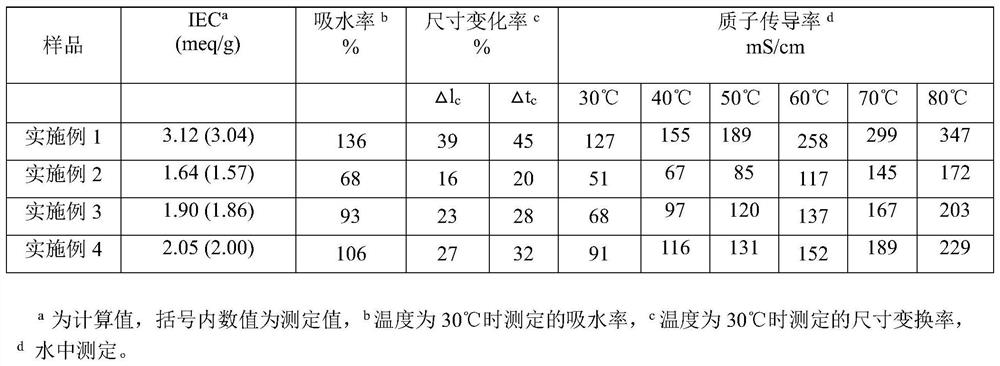
Embodiment 1
[0028] In this embodiment, the tetraamine (4) skeleton is used as an example to synthesize sulfonated polyquinoxaline from the monomer (7) of the polyquinoxaline structural unit (a) before sulfonation, and the structure is as follows:
[0029]
[0030] The specific preparation method is:
[0031] (1) Polymerization
[0032] Weigh 4,4'-bis(2-(4-(4-phenylphenoxy)phenyl)acyl)biphenyl 0.7551g and 2-amino-4-((3,4-diaminophenyl )sulfonyl)aniline 0.2802g, mixed and poured into a flask, added m-cresol to dissolve, reacted at room temperature under nitrogen atmosphere for about 24h, poured the solution into ethanol to precipitate the polymer, washed repeatedly until it was cleaned, filtered, and collected the solid , dried in vacuum to obtain polymer fibers.
[0033] (2) Sulfonation
[0034] Pour the polymer fibers into a flask, add fuming sulfuric acid at a ratio of 10-15wt% solid content, react under a nitrogen atmosphere, pour the liquid into water, the sulfonated product is p...
Embodiment 2
[0038] In this embodiment, the monomer (2) of the polyquinoxaline structural unit (a) and the monomer (3) of the structural unit (b) before sulfonation are used as raw materials to synthesize sulfonated polyquinoxaline with the tetraamine (1) skeleton Taking morphine as an example, the structure is as follows (wherein x:y=4:1):
[0039]
[0040] The specific preparation method is:
[0041] (1) Polymerization
[0042] Weigh 1.2335g of 4,4'-bis(2-(4-(4-phenoxyphenyl)phenyl)acyl)diphenyl ether, 0.1741g of diphenil and 3,3',4,4 0.4285 g of '-tetraaminobiphenyl was reacted in m-cresol. After the reaction, the solution was poured into ethanol to precipitate the polymer, washed repeatedly until cleaned, filtered, and dried to obtain polymer fibers.
[0043] (2) Sulfonation
[0044] React the obtained polymer with concentrated sulfuric acid, pour the solution into water after the reaction, the sulfonated product is precipitated, wash repeatedly until pH=3~4, add appropriate amount...
Embodiment 3
[0048] In this embodiment, the monomer (5) of the polyquinoxaline structural unit (a) and the monomer (3) of the structural unit (b) before sulfonation are used as raw materials to synthesize sulfonated polyquinoxaline with the tetraamine (3) skeleton Taking morphine as an example, the structure is as follows (wherein x:y=4:1):
[0049]
[0050] The specific preparation method is:
[0051] (1) Polymerization
[0052] Weigh 0.9643g of 4,4'-bis(2-(4-phenoxyphenyl)acyl)biphenyl, 0.1739g of 4,4'-benziloxy and 3,3',4,4'- Tetraaminodiphenyl ether 0.4695g was reacted in m-cresol. After the reaction, the solution was poured into ethanol to precipitate the polymer, washed repeatedly until cleaned, filtered, and dried to obtain polymer fibers.
[0053] (2) Sulfonation
[0054] React the obtained polymer with concentrated sulfuric acid, pour the solution into water after the reaction, the sulfonated product is precipitated, wash repeatedly until pH=3~4, add appropriate amount of so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com