Quinoline aromatic ethylene derivative and applications in preparation of fluorescent dyes and fluorescent probes
A quinoline aromatic vinyl, fluorescent dye technology, applied in styryl dyes, fluorescence/phosphorescence, luminescent materials, etc., can solve problems such as small injection volume, achieve low detection limit, low biological toxicity and phototoxicity, fluorescence intensity Enhanced effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
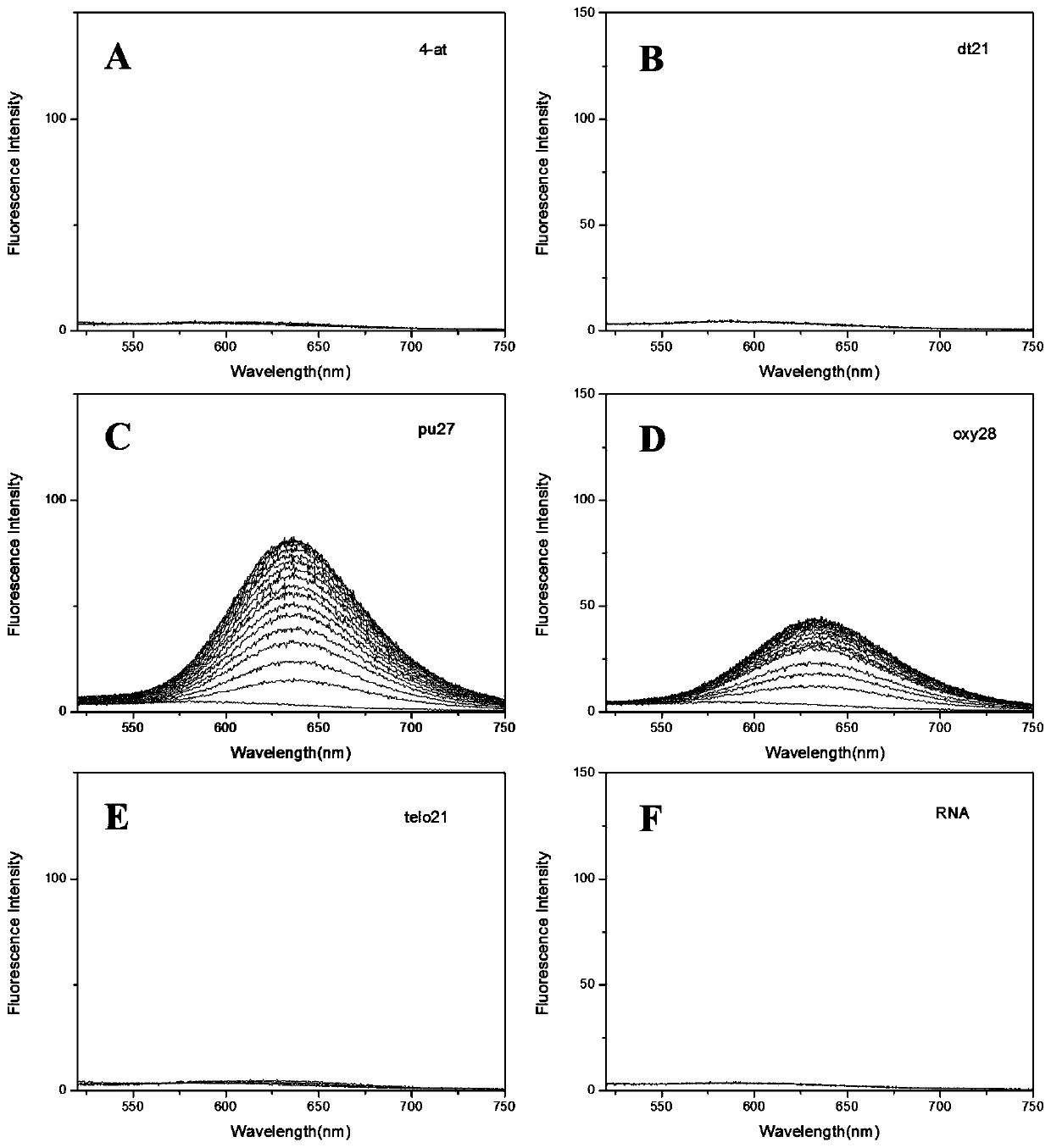
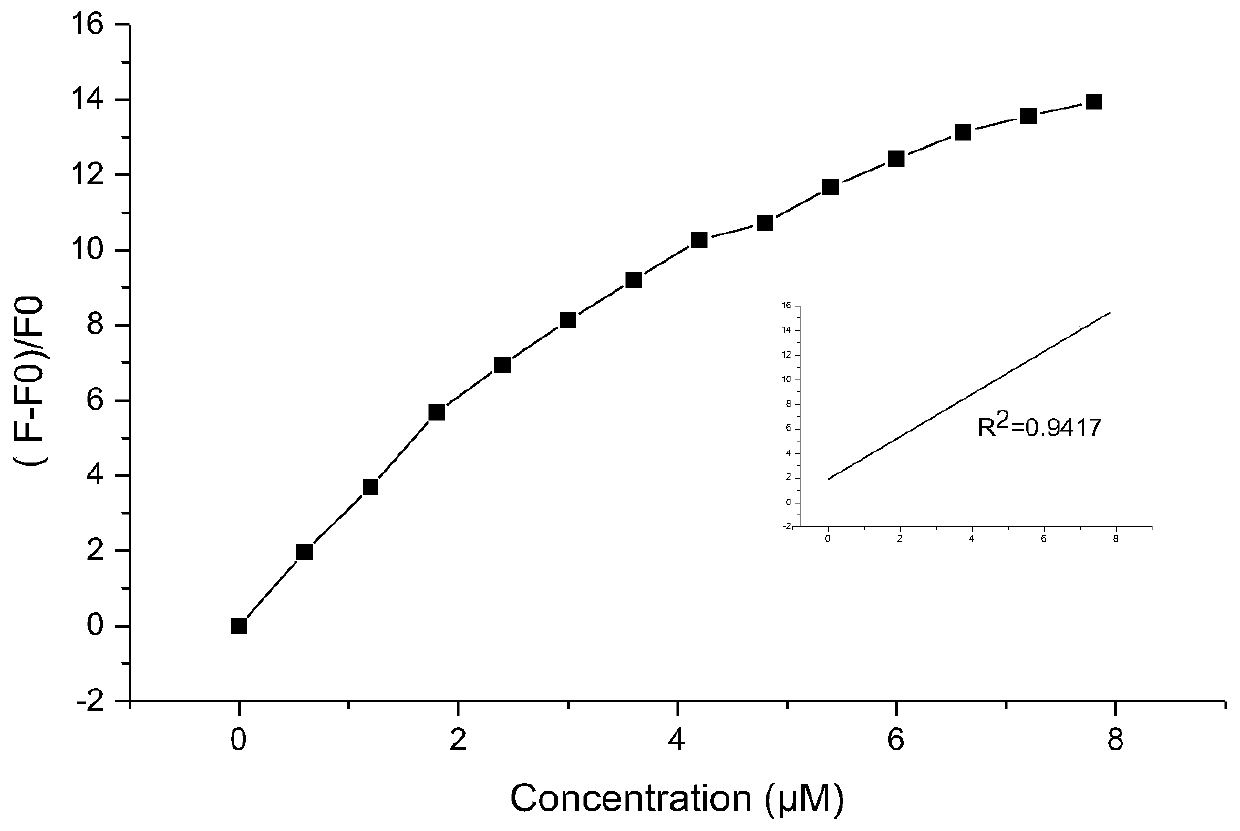
Image
Examples
Embodiment 1
[0052] (1) Dissolve 0.40g (2.28mmol) of 2-methylquinoline in 2.2ml (23.1mmol) of sulfolane, then add dropwise 0.42ml (6.84mmol) of methyl iodide, and then stir at 50°C for 2h; after the reaction After cooling, the reaction solution was poured into 15 mL of ethyl acetate and left to stand, filtered under reduced pressure to obtain the crude product; the crude product was washed with ethyl acetate to obtain 0.53 g of the intermediate product I (1,2-dimethyl-1-iodo Quinoline), the yield is 83%. Its proton nuclear magnetic resonance spectrum results are as follows: 1H NMR (400MHz, DMSO) δ9.13 (d, J = 8.6Hz, 1H), 8.61 (d, J = 9.0Hz, 1H), 8.42 (d, J = 8.0Hz, 1H), 8.27–8.20(m, 1H), 8.15(d, J=8.6Hz, 1H), 8.00(t, J=7.6Hz, 1H), 4.47(s, 3H), 3.12(s, 3H). The synthesis process is as follows:
[0053]
[0054] (2) Mix 0.39g (1.385mmol) of the intermediate product I (1,2-dimethyl-1-iodoquinoline) obtained in step (1) with 4mL (43.7mmol) of n-butanol, and then add 0.282 mL (2.077mmol) ...
Embodiment 2
[0061] (1) Dissolve 0.40g (2.28mmol) of 2-methylquinoline in 2.7mL (28.5mmol) of sulfolane, then dropwise add 0.50mL (8mmol) of methyl iodide, then stir at 60°C for 3h; cool down after the reaction , and then the reaction solution was poured into 24mL of ethyl acetate and left to stand, filtered under reduced pressure to obtain the crude product; the crude product was washed with ethyl acetate to obtain 0.58g of the intermediate product I (1,2-dimethyl-1-iodoquinone phenoline), the yield was 89%. Its proton nuclear magnetic resonance spectrum result is the same as embodiment 1 with synthetic process.
[0062] (2) Mix 0.39g (1.385mmol) of the intermediate product I (1,2-dimethyl-1-iodoquinoline) obtained in step (1) with 4.5mL (49.16mmol) of n-butanol, and then add 0.330mL (2.077mmol) of p-dimethylaminobenzaldehyde and 0.21mL (1.25mmol) of 4-methylpiperidine, then stirred at 45°C for 6.5h; The solid was suction-filtered under reduced pressure to obtain 0.52 g of intermediate ...
Embodiment 3
[0066] (1) Dissolve 0.40g (2.28mmol) of 2-methylquinoline in 3.2mL (34mmol) of sulfolane, then add dropwise 0.57mL (9.12mmol) of methyl iodide, and then stir at 70°C for 4h. Cool after the reaction, then pour the reaction solution into 40mL ethyl acetate and let it stand, filter under reduced pressure to obtain the crude product, wash the crude product with ethyl acetate to obtain 0.57g of the intermediate product I (1,2-dimethyl-1- iodoquinoline), the yield is 87%. Its proton nuclear magnetic resonance spectrum result is the same as embodiment 1 with synthetic process.
[0067] (2) Mix 0.39g (1.385mmol) of the intermediate product I (1,2-dimethyl-1-iodoquinoline) obtained in step (1) with 5.1mL (55.4mmol) of n-butanol, and then add 0.376mL (2.77mmol) of p-dimethylaminobenzaldehyde and 0.23mL (1.385mmol) of 4-methylpiperidine, then stirred at 60°C for 8h; after the reaction, 30mL of petroleum ether was added to the reaction solution, and the solid was precipitated after stand...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com