Application of pyrimidine small-molecule compounds in preparation of drugs with mycobacterium resistance
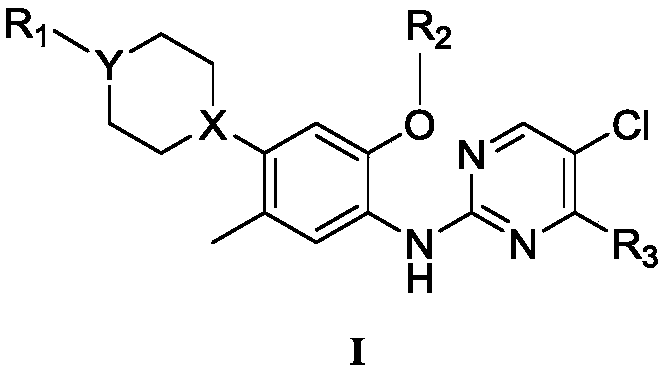
A compound and pyrimidine technology, applied in the field of 2,4-pyrimidinediamine anti-tuberculosis compounds, can solve problems such as difficulty in preventing and treating tuberculosis, liver damage, and difficulty in treating tuberculosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
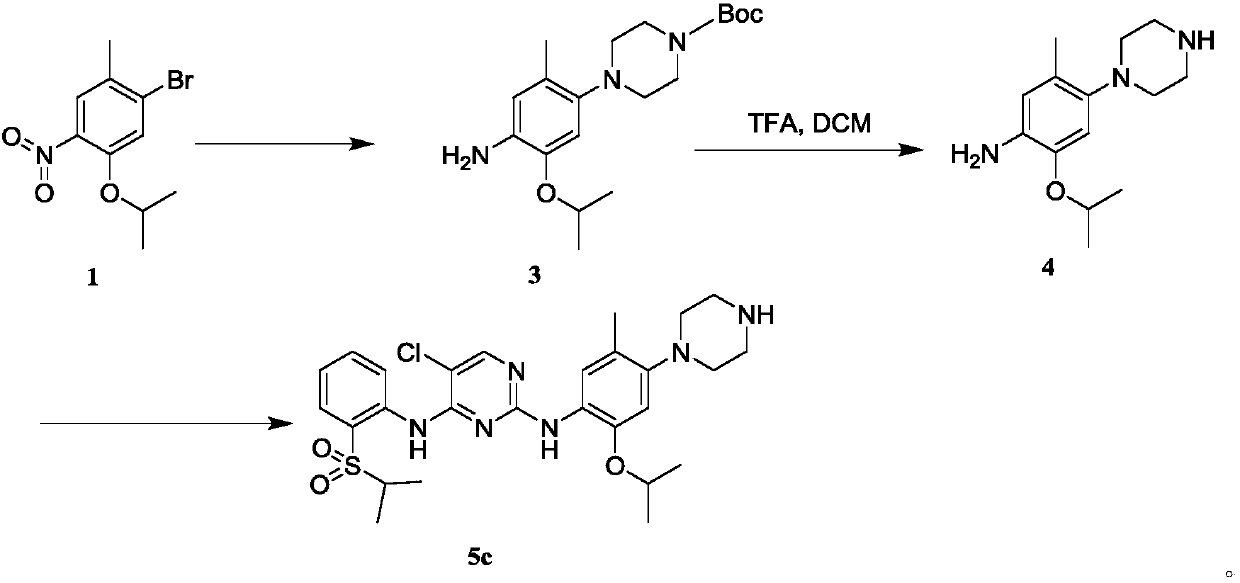
[0065] Example 1: 5-Chloro-N 2 -(2-isopropoxy-5-methyl-4-(4-methylpiperazin-1-yl)phenyl)-N 4 -(2-(sulfonylisopropyl)phenyl)pyrimidine-2,4-diamine (5a)
[0066]
[0067] Step 1 Preparation of 1-(5-isopropoxy-2-methyl-4-nitrophenyl)-4-methylpiperazine (2).
[0068] Add 2.0mmol 1-bromo-5-isopropoxy-2-methyl-4-nitrobenzene (1), 6.0mmol cesium carbonate, 0.8mmol Xantphos reaction substrate, 20.0ml dioxane to a 50mL three-necked flask , 3.0mmolN-methylpiperazine, 0.4mmolPd(AcO) 2 , After N2 replacement for 20min, under the protection of N2, react overnight at 110°C. After the reaction, cool to room temperature, filter, spin dry, and flash column chromatography to obtain the crude product, which is directly put into the next reaction.
[0069] Step 2 Preparation of 2-isopropoxy-5-methyl-4-(4-methylpiperazin-1-yl)aniline (3).
[0070] The 2.0mmol crude product 1-(5-isopropoxy-2-methyl-4-nitrophenyl)-4-methylpiperazine obtained in the previous step, 10mmol iron powder were disso...
Embodiment 2
[0074] Example 2: 5-Chloro-N 2 -(2-Isopropoxy-5-methyl-4-morpholinylphenyl)-N 4 -(2-(sulfonylisopropyl)phenyl)pyrimidine-2,4-diamine (5b)
[0075]
[0076] The synthetic method is as example 1.
[0077] 1 H NMR (400MHz, CDCl 3 )δ9.51(s,1H),8.57(d,J=8.0Hz,1H),8.14(s,1H), 8.01(s,1H),7.93(dd,J=8.0,4.0Hz,1H), 7.68–7.57(m,1H),7.49(s,1H),7.30–7.22(m,1H),6.65(s,1H),4.54(dt,J=12.0,8.0Hz,1H),3.91–3.78( m,4H),3.36–3.15(m,1H),2.94–2.81(m,4H),2.15(s,3H),1.38(d,J=4.0Hz,6H),1.32(d,J=8.0Hz ,6H). 13 C NMR (101MHz, DMSO-d 6)δ157.24,155.42,45.72,145.10,138.44, 134.64,131.28,124.96,124.69,123.68,123.19,121.61,105.66,105.60,71.85,67.47 (2C),55.49,52.56(2C),22.26(2C),17.37, 15.37(2C).HRMS for C 27 h 34 ClN 5 o 4 S calcd, 559.2020; found, 560.2098 (M+H + ).
Embodiment 3
[0078] Example 3: 5-Chloro-N 2 -(2-Isopropoxy-5-methyl-4-(piperazin-1-yl)phenyl)-N 4 -(2-(sulfonylisopropyl)phenyl)pyrimidine-2,4-diamine (5c)
[0079]
[0080] The synthesis method for synthesizing intermediate 3 from starting materials is as in steps 1-2 in Example 1.
[0081] Wherein the synthetic steps of intermediate 4 are as follows:
[0082] Intermediate 3 (0.4mmol) was dissolved in dichloromethane, 2.0mmol TFA was added under stirring, and the reaction was carried out at room temperature for 4h. After the reaction, the organic solvent was spin-dried to obtain a crude product, which was directly put into the next reaction.
[0083] The synthesis method of the final product 5c from the intermediate 4 is as step 3 in Example 1.
[0084] 1 H NMR (400MHz, CDCl 3 )δ9.53(s,2H),8.54(d,J=8.0Hz,1H),8.16(s,1H),8.05(s,1H),7.93(dd,J=8.0,4.0Hz,1H), 7.80(d,J=8.0Hz,1H),7.65–7.57(m,1H), 7.54(s,1H),7.30–7.21(m,1H),6.65(s,1H),4.53(dt,J= 12.0,8.0Hz,1H),3.43(s,4H),3.26(dt,J=12.0,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com