Medical degradable hemostatic material and preparation method thereof
A hemostatic material and soluble technology, which is applied in the field of new medical degradable hemostatic material and its preparation, can solve the problems of epichlorohydrin crosslinking agent toxicity, high price, adhesion, and insufficient liquid absorption, etc., to improve the product Safety, rapid mass absorption, and easy-to-operate effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1 Preparation of medical degradable hemostatic material
[0041] 1. Self-crosslinking: Weigh 10g of chitosan and 20g of sodium starch glycolate and mix them well. After mixing, dry heat treatment at 120°C for 2 hours to obtain a crosslinked sample.
[0042] 2. Preparation of microspheres: Dissolve the cross-linked sample in 1000ml of water, stir and dissolve, and add the solution dropwise to 20% calcium chloride solidified solution by electrostatic droplet method, the voltage is 20kV, and the feeding rate is 300μL / min.
[0043] 3. Microsphere pore making: put the microsphere solution into an ultrasonic instrument for normal temperature ultrasonication, ultrasonic frequency 40kHz, power 1000W, ultrasonication for 20min.
[0044] 4. Drying: The porous microspheres are dried by means of supercritical drying. The conditions of supercritical drying are: temperature 45°C, vacuum degree 8.5MPa.
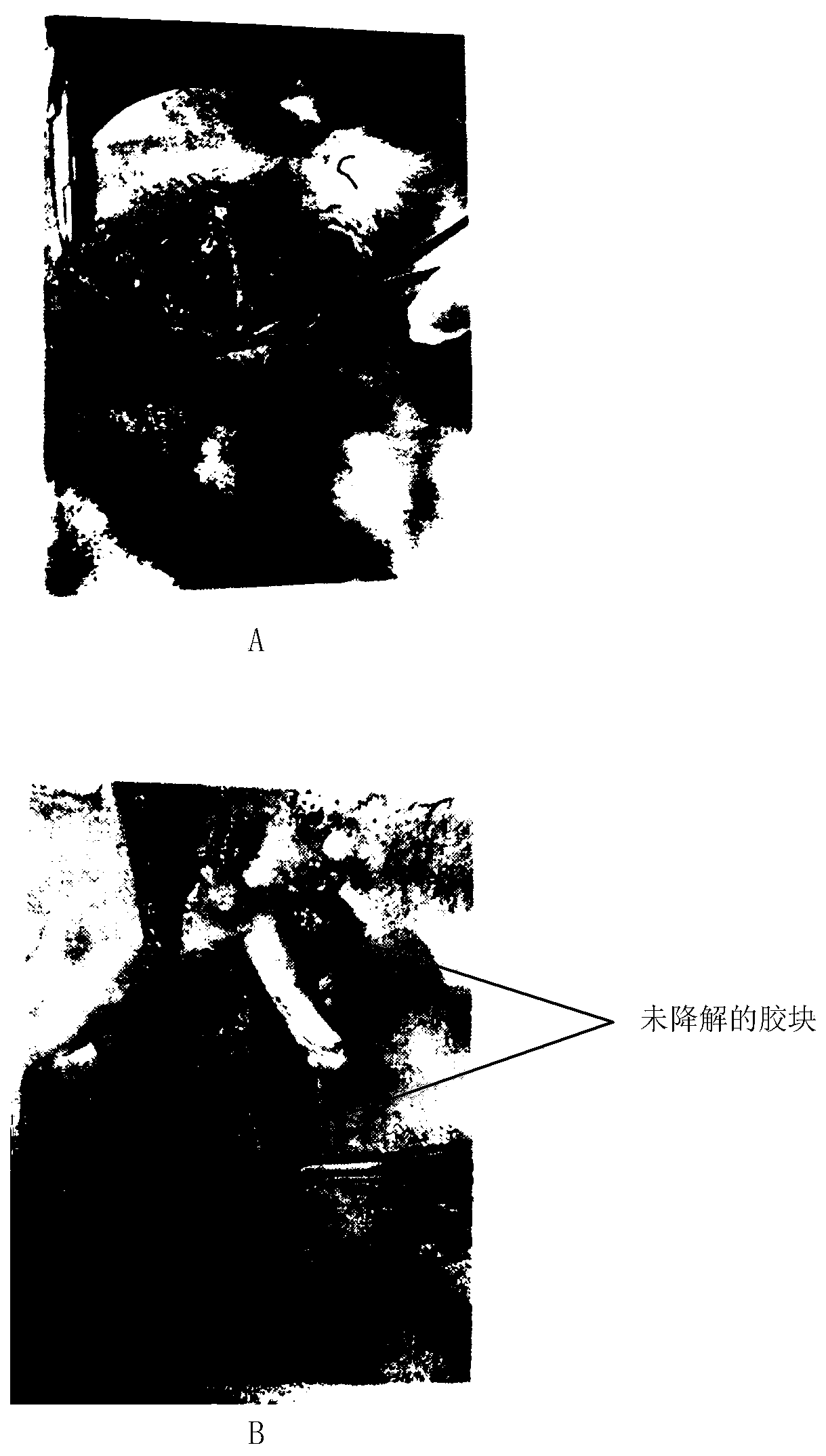
[0045] 5. Sterilization: The above samples are packaged and sterili...
Embodiment 2
[0046] Embodiment 2 Preparation of medical degradable hemostatic material
[0047] 1. Self-crosslinking: Weigh 10g of carboxymethyl chitosan and 20g of carboxymethyl starch sodium and mix evenly, dry heat treatment at 135°C for 4 hours to obtain a crosslinking sample.
[0048] 2. Preparation of microspheres: Dissolve the cross-linked sample in 2000ml of water, stir and dissolve, and add the solution dropwise to 15% calcium chloride solidified solution by electrostatic droplet method, the voltage is 40kV, and the feeding rate is 200μL / min.
[0049] 3. Microsphere pore making: put the microsphere solution into an ultrasonic instrument for normal temperature ultrasonication, ultrasonic frequency 28kHz, power 1000W, ultrasonic 40min.
[0050] 4. Drying: The above-mentioned porous microspheres are dried by spray drying.
[0051] 5. Sterilization: The above samples are packaged and sterilized to obtain a medical degradable hemostatic material.
Embodiment 3
[0052] Embodiment 3 Preparation of medical degradable hemostatic material
[0053] 1. Self-crosslinking: Weigh 5g of carboxymethyl chitosan and 20g of pregelatinized hydroxypropyl starch, mix evenly, and dry heat at 150°C for 3 hours to obtain a crosslinked sample.
[0054] 2. Preparation of microspheres: Dissolve the cross-linked sample in 800ml of water, stir and dissolve, and add the solution dropwise to 10% calcium chloride solidified solution by electrostatic droplet method, the voltage is 20kV, and the feeding rate is 300μL / min.
[0055] 3. Microsphere pore making: put the microsphere solution into an ultrasonic instrument for normal temperature ultrasonication, ultrasonic frequency 40kHz, power 1000W, ultrasonic 30min.
[0056] 4. Drying: The above-mentioned porous microspheres are dried by spray drying.
[0057] 5. Sterilization: The above samples are packaged and sterilized to obtain a medical degradable hemostatic material.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com