Crisaborole intermediate preparation method
A technology of crisborole and intermediates, applied in the field of pharmacy, can solve the problems of high price of 2-bromo-5-hydroxybenzaldehyde, increase of preparation cost of crisborole intermediates, low selectivity of bromination reaction, etc. Achieve the effects of cheap raw materials, improved bromination reaction selectivity, and short preparation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
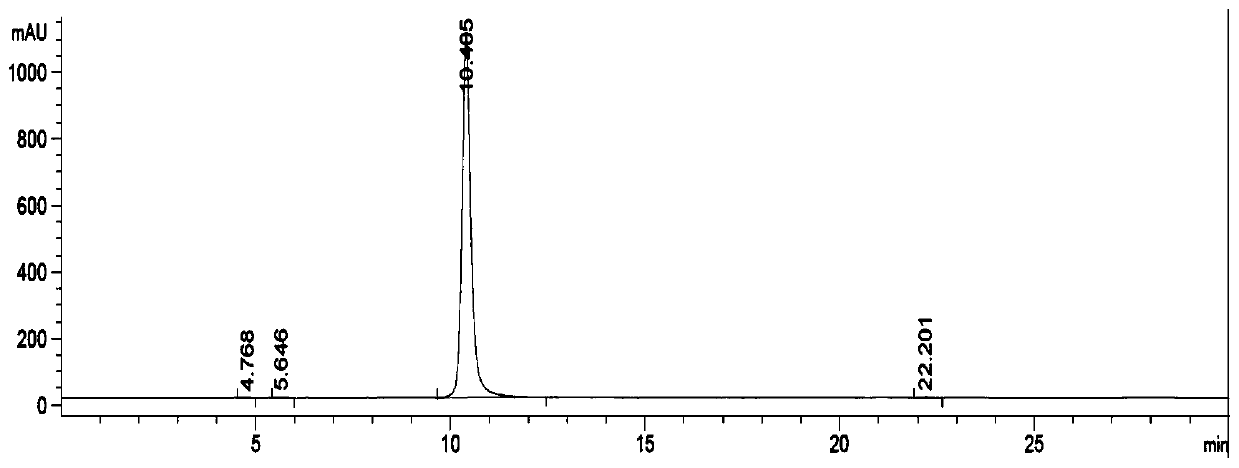
Image
Examples
preparation example Construction
[0024] The invention provides a method for preparing a crisborole intermediate, the crisborole intermediate has the structure shown in formula IV,
[0025] This preparation method comprises the steps:
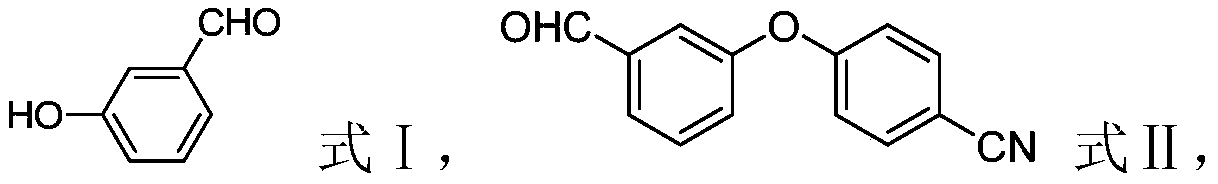
[0026] (1) In the presence of the first organic solvent and the first base, the compound shown in formula I is contacted with p-fluorobenzonitrile to obtain the compound shown in formula II;
[0027]
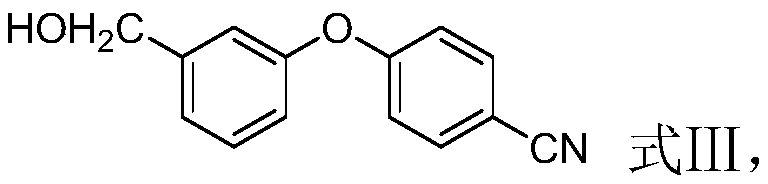
[0028] (2) In the presence of a second solvent, the compound represented by the formula II and the alkali metal borohydride are subjected to a contact reaction to obtain the compound represented by the formula III;
[0029]
[0030] (3) In the presence of a third organic solvent and a catalyst, the compound represented by the formula III and the bromination reagent are subjected to a contact reaction to obtain the compound represented by the formula IV;
[0031]
[0032] According to the present invention, preferably, in step (1), the first organic solvent is at least o...
Embodiment 1
[0047] As shown in Formula 3, this embodiment provides a preparation method of the crisborole intermediate, and the specific preparation method is as follows:
[0048] (1) Preparation of 4-(3-(formyl)phenoxy)benzonitrile (compound shown in formula II)
[0049] Add 122g (1mol) of m-hydroxybenzaldehyde (compound shown in formula I), 400ml of DMF, and 276g (2mol) of potassium carbonate into the reaction kettle, add 182g (2mol) of p-fluorobenzonitrile under stirring, react at 110°C for 16h, cool, Add 2000ml of water, extract with ethyl acetate three times, recover ethyl acetate, beat with methanol, filter, and dry to obtain 203g of brown solid, yield 91%;
[0050] (2) Preparation of 4-(3-(hydroxymethyl)phenoxy)benzonitrile (compound shown in formula III)
[0051] 223g (1mol) of 4-(3-(formyl)phenoxy)benzonitrile (compound shown in formula II), 2000ml methanol was added to the reaction kettle, and then 18.9g (1mol) sodium borohydride was added to the reaction in batches Kettle, af...
Embodiment 2
[0054] As shown in Formula 3, this embodiment provides a preparation method of the crisborole intermediate, and the specific preparation method is as follows:
[0055] (1) Preparation of 4-(3-(formyl)phenoxy)benzonitrile (compound shown in formula II)
[0056]Add 122g (1mol) of m-hydroxybenzaldehyde (compound shown in formula I), 400ml of DMF, and 276g (2mol) of potassium carbonate into the reaction kettle, add 182g (2mol) of p-fluorobenzonitrile under stirring, react at 110°C for 16h, cool, Add 2000ml of water, extract with ethyl acetate three times, recover ethyl acetate, beat with methanol, filter, and dry to obtain 203g of brown solid, yield 91%;
[0057] (2) Preparation of 4-(3-(hydroxymethyl)phenoxy)benzonitrile (compound shown in formula III)
[0058] 223g (1mol) of 4-(3-(formyl)phenoxy)benzonitrile (compound shown in formula II), 2000ml methanol was added to the reaction kettle, and then 18.9g (1mol) sodium borohydride was added to the reaction in batches Kettle, aft...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com