A kind of cobalt complex and its preparation method and application as proton conduction material
A technology of cobalt complexes and ligands is applied in the application field of cobalt complexes as proton conductive materials, which can solve problems such as insufficient understanding of electrical conductivity, and achieve the effects of excellent proton conductivity, simple operation and simple preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
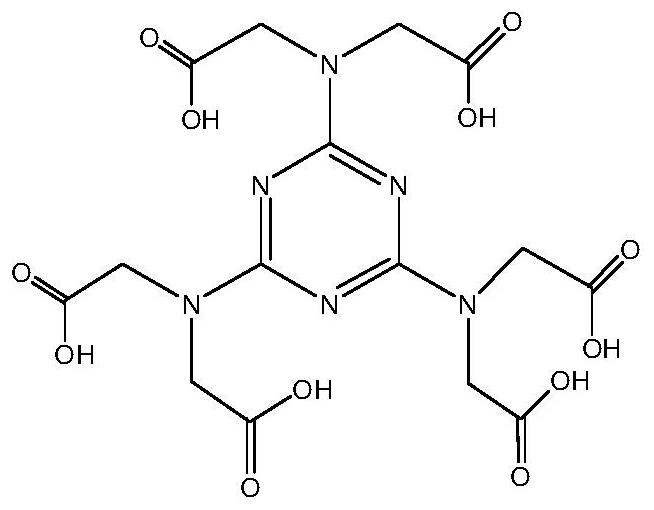
[0046] 0.4g H 6 TTHA, 0.64g 4,4′-bipyridine and 0.8g Co(NO 3 ) 2 ·6H 2 O was added to 25 mL of deionized water. Then place it in an autoclave at 100° C. for 48 hours at a constant temperature. After cooling down to room temperature, crystals are precipitated, and the crystals are washed with deionized water for several times to obtain a cobalt complex. The cobalt complex is a pink blocky crystal. Elemental analysis data C 80 h 110 N 22 o 44 co 3 , Theoretical: C, 42.5; H, 4.9; N, 13.63%. Found: C, 42.9; H, 4.54; N, 13.25%. Main infrared data (KBr, cm -1 ): 3259(s); 2940(w); 1704(s); 1605(m); 1549(m); 1482(s); 1318(s).
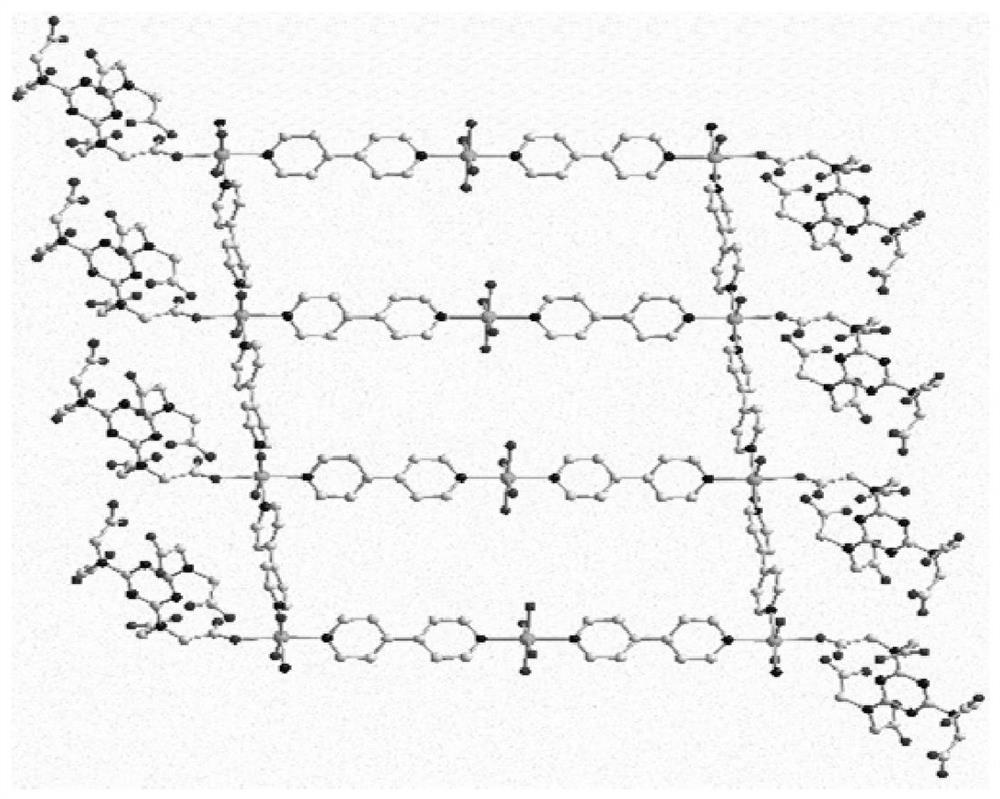
[0047] Analyze the single crystal structure of the cobalt complex prepared in Example 1 of the present invention, collect the single crystal diffraction data with a Bruker Smart CCD diffractometer, and use a graphite monochromator to monochromatize Mo / kα rays Scan, obtain following result: the cobalt complex that the embodiment of the present in...
Embodiment 2
[0049] 0.4g H 6 TTHA, 0.64g 4,4′-bipyridine and 0.8g Co(NO 3 ) 2 ·6H 2 O was added to 25 mL of deionized water. Then place it in an autoclave at 140° C. for 72 hours at a constant temperature. After cooling to room temperature, crystals precipitate out. The crystals are washed with deionized water several times to obtain a cobalt complex. The cobalt complex is a pink blocky crystal.
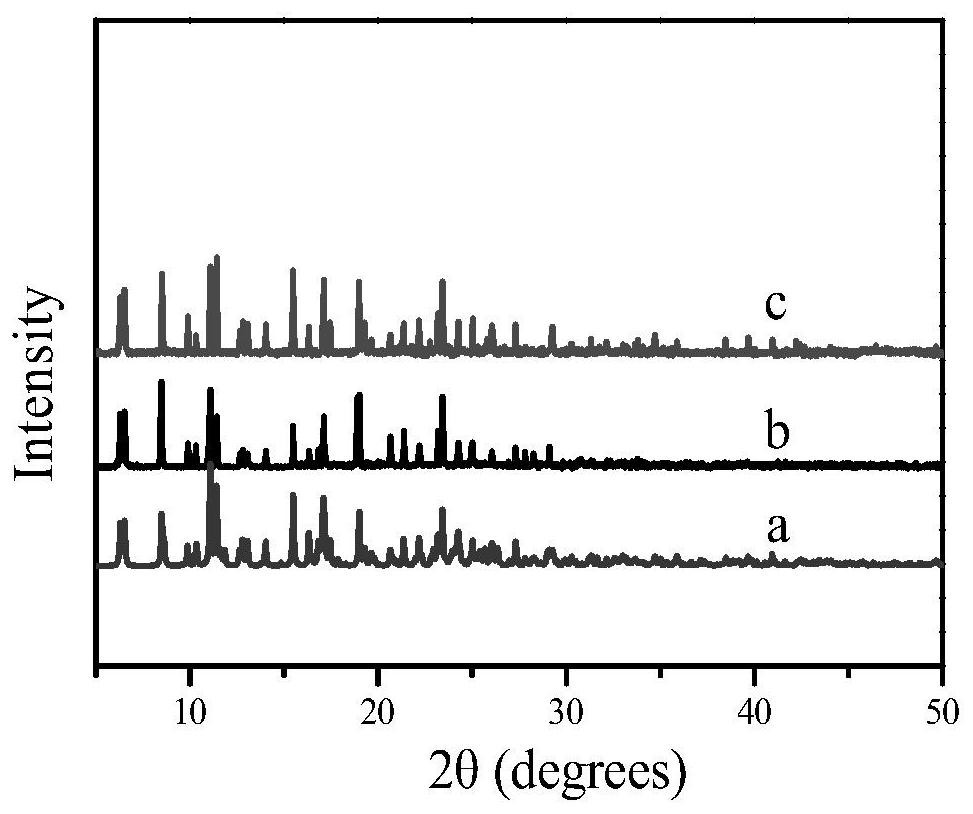
[0050] The cobalt complex prepared in Example 2 of the present invention was analyzed by X-ray diffraction, and its diffraction peaks were consistent with the simulated diffraction peaks obtained by single crystal analysis in Example 1 of the invention, such as image 3 As shown, it is illustrated that the same substance is obtained according to the preparation methods of Example 1 and Example 2.
[0051] In order to investigate the proton conductivity of the cobalt complex prepared in the present invention, about 50 mg of the cobalt complex prepared in Example 2 of the present invention was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com