Fully human anti-Staphylococcus aureus α-hemolysin recombinant antibody
A Staphylococcus, recombinant antibody technology, applied in the direction of recombinant DNA technology, anti-bacterial immunoglobulin, immunoglobulin, etc., can solve the problem of lack of fully human antibodies, achieve strong penetration, small molecular weight, and retain biological Effects of Activity and Specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation of embodiment 1 Staphylococcus aureus recombinant protein antigen
[0029] A large amount of antigenic protein is required in the process of preparing fully human antibody against Staphylococcus aureus α-HL. In order to promote the soluble expression of the antigenic protein, p-ColdTF fusion expression vector is used for expression, which carries TF molecular chaperone (48KD), which is beneficial to the co-translational folding of the newly expressed polypeptide. In addition, the vector carries a polyhistidine tag (His-Tag), which is beneficial for protein purification.
[0030] Boiling method to extract total RNA of Staphylococcus aureus, using total RNA as template, Oliga dT 15 cDNA was obtained by reverse transcription with primers. The α-HL gene sequence of Staphylococcus aureus was obtained from the NCBI gene database, primers were designed according to the gene sequence, and α-HL was amplified by nested PCR. The synthetic recombinant protein gen...
Embodiment 2
[0032] Example 2 Using phage display antibody library technology to screen anti-Staphylococcus aureus α-HL single chain antibody
[0033] Our laboratory has constructed a natural fully human scFv antibody library in the early stage, with a library capacity of 2.5×10 8 , good diversity. Using α-HL biotinylated protein as antigen, the natural fully human scFv antibody library was enriched by phage display by immunomagnetic bead method, clones were randomly selected from the enriched scFv antibody library, and monoclonal phage amplification was carried out. The expressed scFv was detected by phage ELISA with anti-M13-HRP monoclonal antibody. For the specific experimental steps of this example, see: Master's degree thesis "Wu Tong. Cloning and expression of Staphylococcus aureus α-hemolysin and screening of anti-HLA-α fully human single-chain antibody [D]. Sichuan: Sichuan Medical University, 2016".
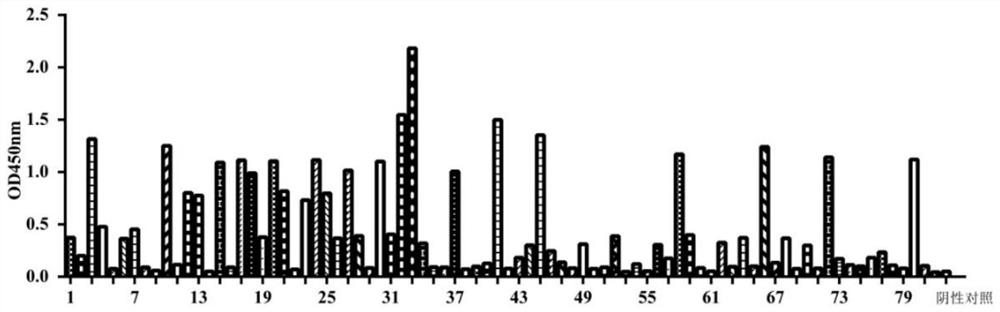
[0034] The results showed that after four rounds of phage display screening, ...
Embodiment 3
[0035] Example 3 Preliminary identification of anti-α-HL single-chain antibody
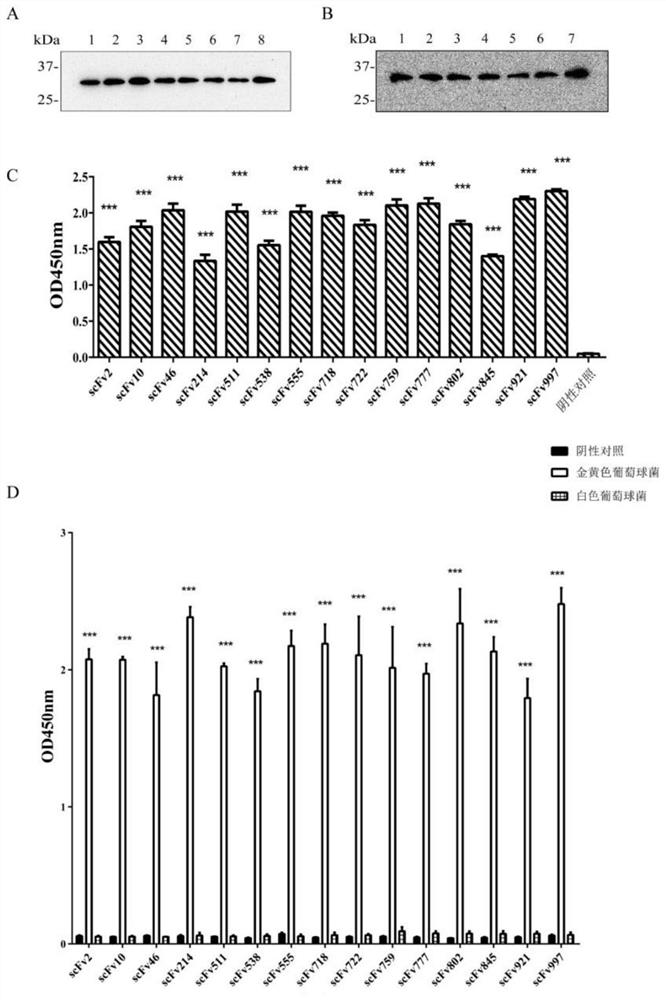
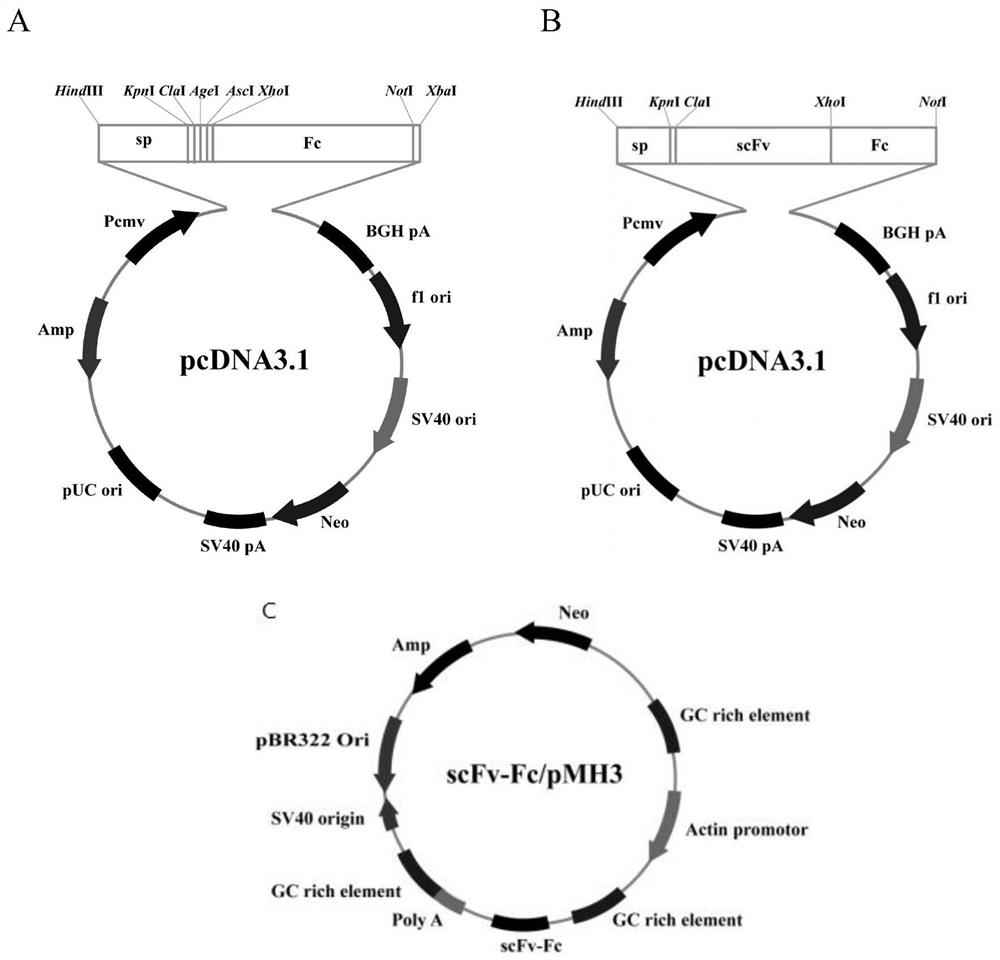
[0036] The OD obtained from the natural fully human scFv antibody library in Example 2 450 The single-chain antibody with the highest value was expressed in large quantities, and the plasmid was extracted and sequenced according to the instructions. The 15 strains of anti-α-HL single-chain antibody with correct sequencing results were inserted into the prokaryotic expression vector pLZ16 for soluble expression verification. The pLZ16 vector was constructed by our laboratory based on the pUC plasmid, containing FLAG and His-tag tags, which have been reported in some documents published by our laboratory, such as "Wang Xu, Yuan Qing, Ye Yingchun, etc. Anti-IL-33 whole human Soluble expression and identification of source scFv-Fc antibody [J] Modern Immunology, 2016; 36(6): 462-465".
[0037] 1. PCR amplification of scFv target gene and verification
[0038] (1) Preparation of reaction solution (20...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com