Chaetomium thermophilum temperature-resistant catalase and application
A technology of catalase and thermophilic Chaetomium, applied in the field of genetic engineering, can solve the problems of poor high temperature resistance of CAT, achieve good high temperature and high humidity resistance, accelerate popularization and application, and reduce market price Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
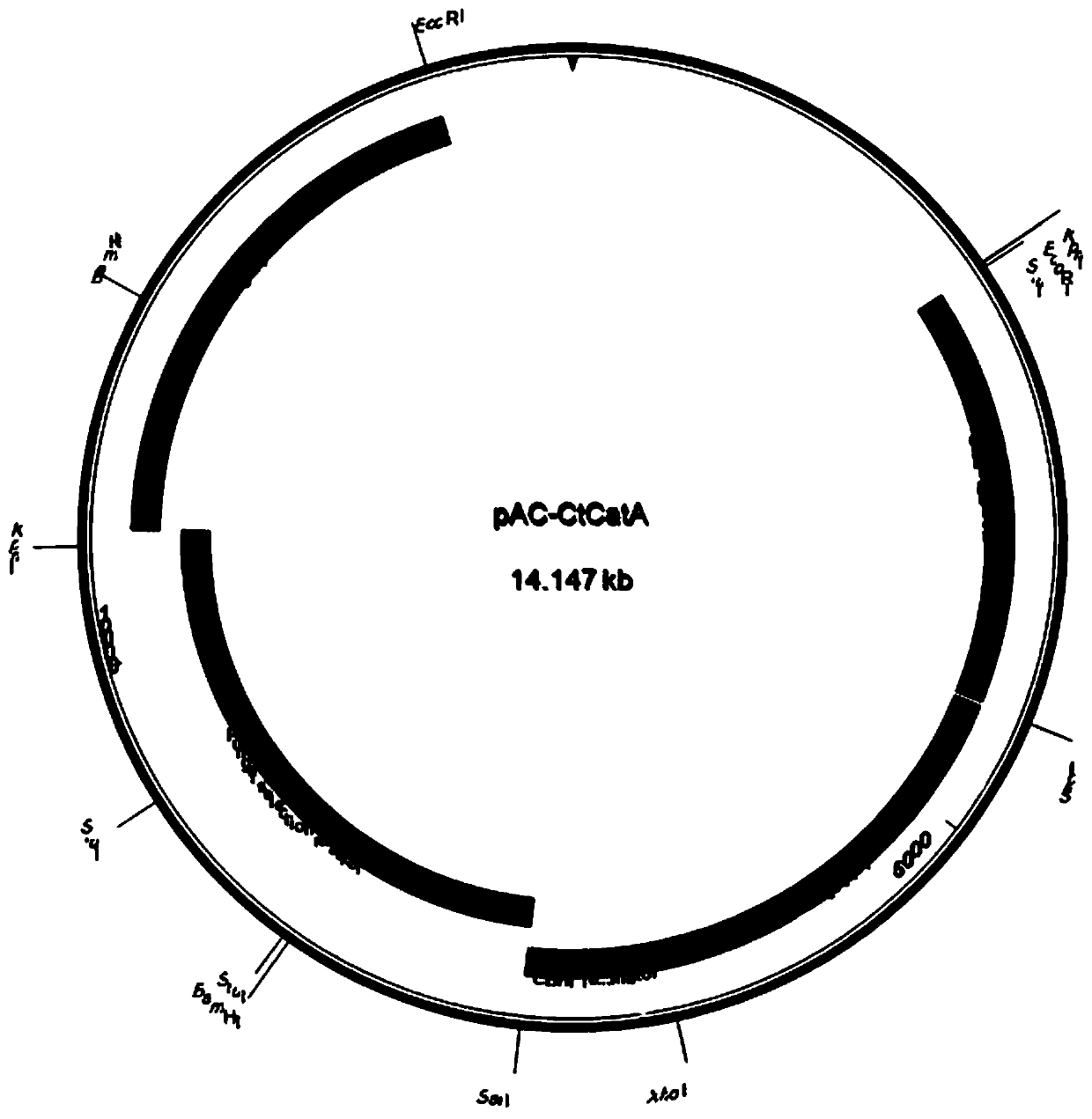
[0027] 1. Discovery of the CatA gene of Chaetomium thermophiles
[0028] By using the amino acid sequence of Aspergillus niger (Aspergillus niger) catalase Cat-R (Fowler et al., 1993) as a template, a search for Chaetomiumthermophilum DSM1495 in the US Center for Biotechnology Information (NCBI) showed that there are three Genes with unknown functions (accession numbers CTHT_0024820, CTHT_0021320 and CTHT_0030860) have relatively low homology (33-57%), and the amino acid residue numbers of these three proteins are 723, 776 and 583, respectively.
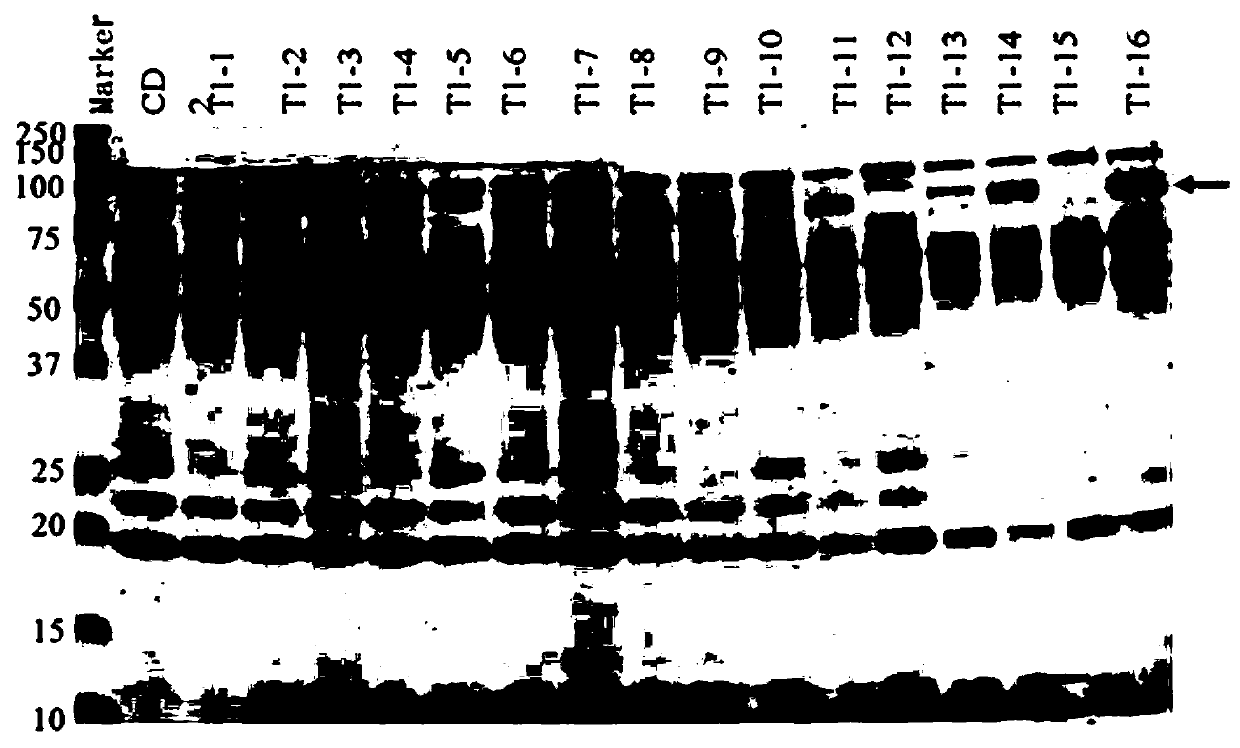
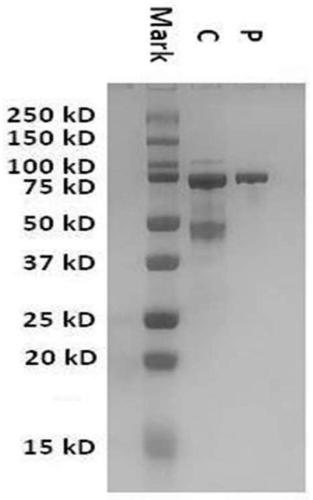
[0029] 2. Expression and secretion of Chaetomium thermophilia CatA gene in Trichoderma
[0030]The protein and corresponding gene of Chaetomium thermophila are respectively named as CatA and catA, and the amino acid sequence is shown in SEQID No.1, which contains 2172 codon reverse transcriptions according to the highly expressed gene of Trichoderma reesei The deoxyribonucleic acid (DNA) sequence of the base pair, in order to facili...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com