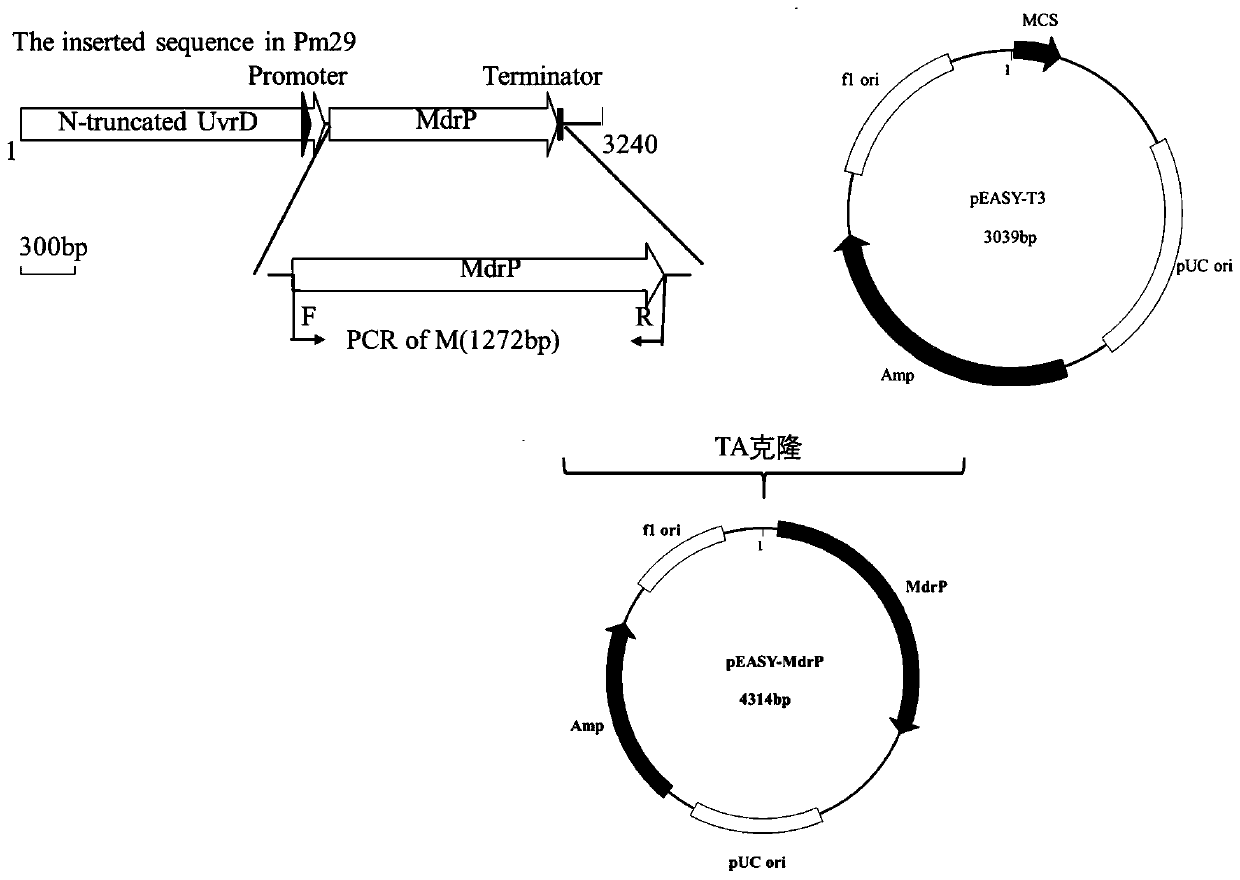
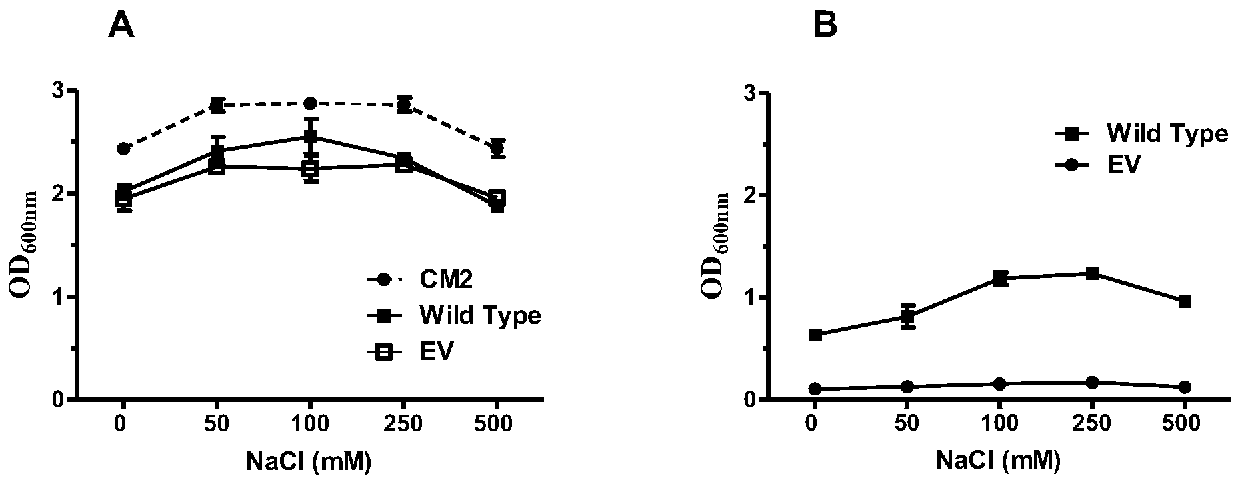
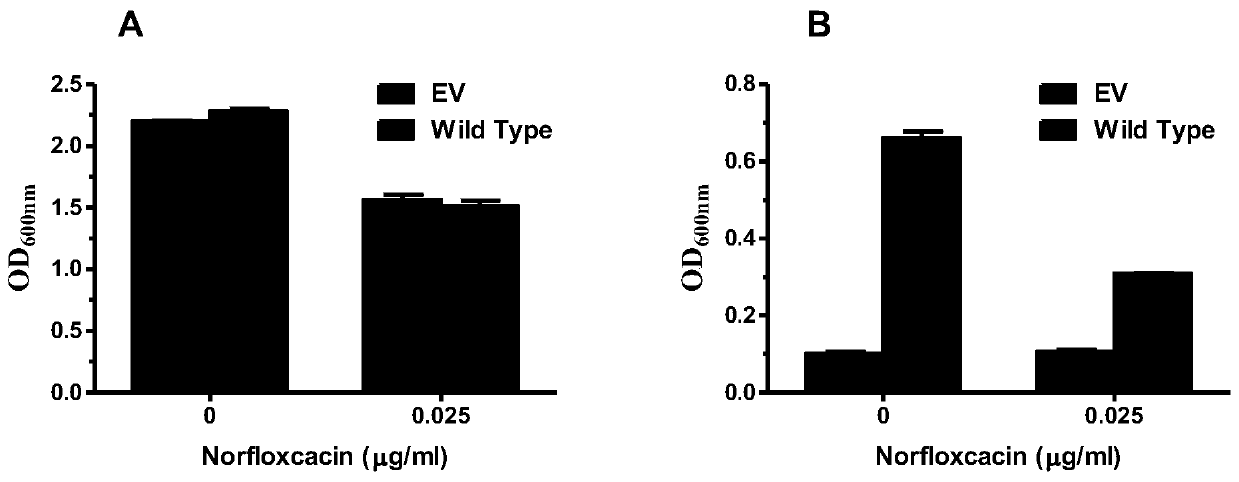
MdrP mutant gene, amino acid, and protein function and drug accumulation activity detection
A technology of mutants and proteins, applied in the field of proteins, can solve problems such as difficult to achieve
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0047] In order to make the object, technical solution and advantages of the present invention clearer, the present invention will be further described in detail below in conjunction with the examples. It should be understood that the specific embodiments described here are only used to explain the present invention, not to limit the present invention.
[0048] Measuring norfloxacin drug accumulation experiment in the prior art can only confirm that the protein is active and transmembrane H + Gradients can serve as driving forces, but other cations (Na + 、K + )'s effect, not to mention H + Relatively quantitative comparative analysis of the driving effect, the more commonly used method for different ion binding is to explain at the protein level, but it is impossible to determine the binding and effectively play a role in transport in a single experiment, and the experiment is time-consuming and laborious .
[0049] In order to solve the above technical problems, the prese...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com