An improved process for the preparation of boronic acid esters
A compound, selected technology, applied in the field of preparing crystalline forms of bortezomib, which can solve the problems of being unsuitable for commercial scale, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0075] Salts of compounds may be prepared according to general standard methods of salt preparation known in the art.
[0076] As used herein, the term "single-phase solvent system" refers to a solvent system that uses only a single solvent during a reaction.
[0077] The term "boronic acid acceptor" as used herein refers to an agent that promotes the cleavage of the ester bond of the boronic acid moiety and releases pinanediol. Those skilled in the art are familiar with the selection of boronic acid acceptors and will appreciate that many different boronic acid acceptors are known in the art such as isobutylboronic acid, isopropylboronic acid, 2-methyl-1-propylboronic acid, boronic acid acceptor The suitability of the individual or otherwise depends on the specific synthetic scheme of the scheme.
[0078] The term "compound of formula I free of deboronation and dimer impurities" refers to a compound of formula I in which these impurities are not detected by HPLC measurement....
Embodiment
[0141] In order to demonstrate the benefit of this specification, an example of the prior art was performed and shown as a reference example.
[0142] X-Ray Powder Diffraction (XRPD): XRPD analysis was performed on a Panalytical, Model-Empyrean X-Ray powder diffractometer. The instrument parameters are as follows:
[0143]
[0144] Use (Chiralpak AD-H post, measure chiral purity by HPLC, parameter is as follows:
[0145]
[0146]
Embodiment -1
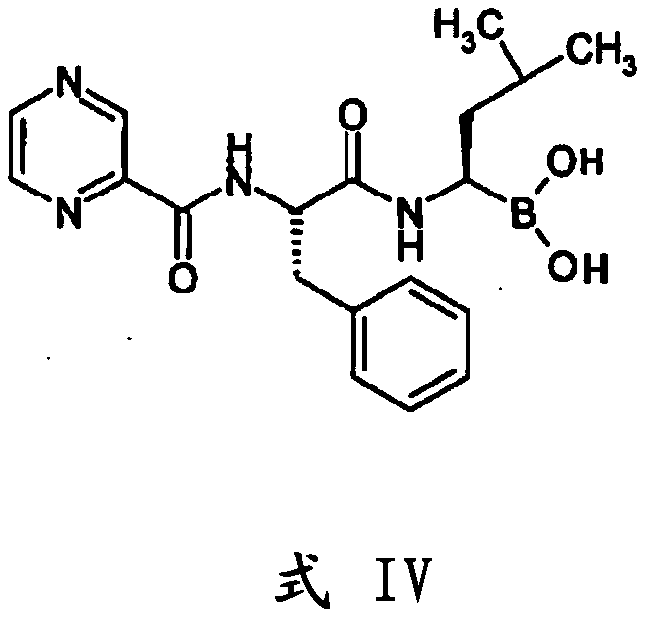
[0148] Preparation of (1S, 2S, 3R, 5S)-pinanediol N-BOC-L-phenylalanine-L-leucine boronate (formula I)
[0149] Boc-L-phenylalanine (100 g) was added to a mixture of acetonitrile (1000 mL), diisopropylethylamine (146.1 g) and TBTU (145.2 g) at a temperature range of -10 to 5 degrees Celsius. The trifluoroacetate salt of pinanediol-leu-boronate (143 g) was added and the reaction mixture was stirred at a temperature ranging from -5 to 5°C for 2 hours.
[0150] Distilled water (1500 mL) was added to the reaction mixture at 10 to 30 degrees Celsius and stirred at 20 to 30 degrees Celsius for 1-2 hours. The resulting solid was filtered, washed with distilled water (2 x 400 mL) and dried in vacuo. The solid was recrystallized from a mixture of acetonitrile and water.
[0151] Yield: 93.3% (180g)
[0152] HPLC purity: ~99.8%
PUM
| Property | Measurement | Unit |
|---|---|---|
| chiral purity | aaaaa | aaaaa |
| chiral purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com