One-step triple real-time fluorescent quantitative PCR detection primer and probe for SVA, type O FMDV and type A FMDV
A real-time fluorescence quantitative and SVA-F technology, applied in the field of animal disease pathogen detection, can solve the problems of indistinguishability, difficulty in clinical diagnosis, and inability to quickly distinguish raw materials for vaccine production, etc., achieving broad application prospects and reducing workload and cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: Design detects primers and TaqMan probes of Seneca virus, O-type foot-and-mouth disease virus and A-type foot-and-mouth disease virus with real-time fluorescent quantitative PCR technology
[0045] From the NCBI nucleic acid database GenBank (http: / / www.ncbi.nlm.nih.gov), the sequence of the whole genome of Seneca virus, the sequence of the whole genome of type O foot-and-mouth disease virus and type A foot-and-mouth disease virus (GeneBank number: NC_011349 、 KT321458、KX173339、KX173338、KX173340、KX751943、KX751944、KY747510、KY038016、 KY747511、KY747512、KX751945、KX751946、KX377924、KY419132、DQ641257、KU051392、 KT757280、KU051391、KY486158、KY486165、KC667560、KR063109、KR063107、KY368743、 AF506822 .2, KX712091.1, HQ412603.1, AJ539141.1, AY390432.1, AY304994.1, GQ406249.1, KT968663.1, HQ632773.1, KY421683.1, KY421686.1, KY07763.210.68, KY07763.210.64 , KF450794.1, AJ131665.1), compared with DNA Star software, according to the design principles of primers and TaqMan probe...
Embodiment 2
[0075] Embodiment 2: Triple real-time fluorescent quantitative PCR detection of Seneca virus, O-type foot-and-mouth disease virus and A-type foot-and-mouth disease virus
[0076] 1. Genomic RNA extraction of Seneca virus, type O foot-and-mouth disease virus and type A foot-and-mouth disease virus
[0077] With Seneca virus cell culture (Jinyu Baoling company vaccine strain, for obtaining standard substance and positive control substance), O type foot-and-mouth disease virus cell culture (Jinyu Baoling company vaccine strain, for obtaining standard substance and Positive control substance) and type A foot-and-mouth disease virus cell culture (Jinyu Baoling company vaccine strain, used to obtain standard substance and positive control substance) and the sample to be tested as the sample to be extracted, extract the genomic RNA of the sample to be extracted, and specifically extract The method refers to the introduction of AXYGEN kit (AxyprepTMBody Fluid Viral DNA / RNA Miniprep Ki...
Embodiment 3
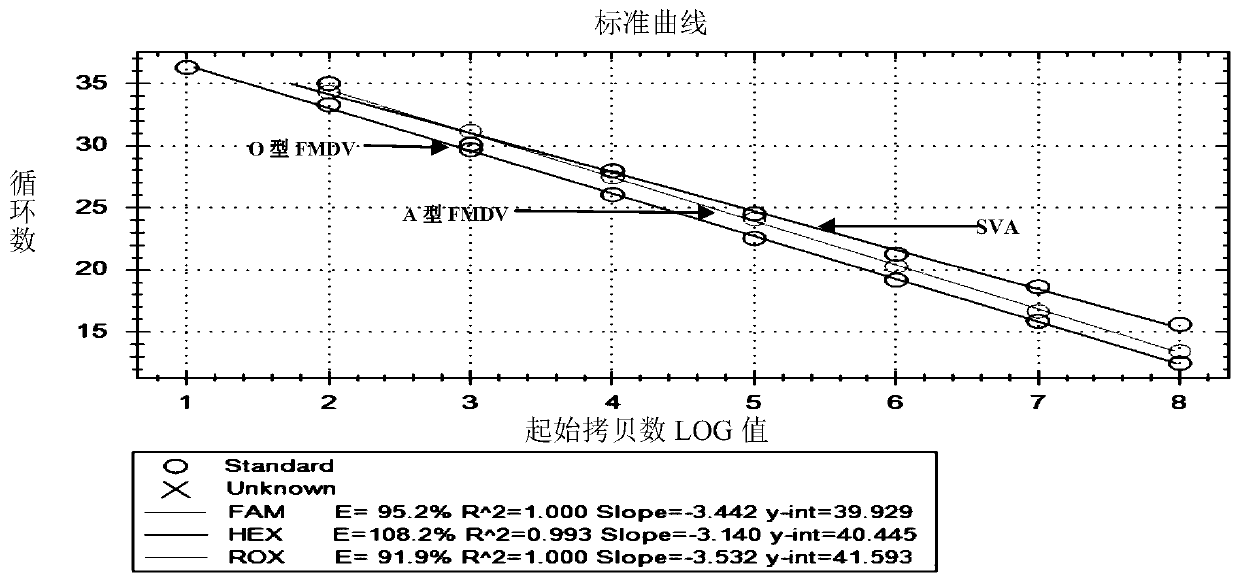
[0119] Example 3, Sensitivity, specificity and repeatability of the triple real-time fluorescent quantitative PCR detection method for Seneca virus, O-type foot-and-mouth disease virus and A-type foot-and-mouth disease virus of the present invention
[0120] 3.1. Sensitivity detection
[0121] Recombinant plasmids pCR-4TOPO-SVA, pCR-4TOPO-O-FMDV and pCR carrying the Seneca virus nucleotide detection gene, O-type foot-and-mouth disease virus nucleotide detection gene and A-type foot-and-mouth disease virus nucleotide detection gene respectively -4 TOPO-A-FMDV was used as a standard (see Example 2), and was diluted to 3×10 according to a 10-fold gradient 8 , 3×10 7 , 3×10 6 , 3×10 5 , 3×10 4 , 3×10 3 , 3×10 2 , 3×10 1 copies / μL were mixed to obtain a standard concentration of 1×10 8 , 1×10 7 , 1×10 6 , 1×10 5 , 1×10 4 , 1×10 3 , 1×10 2 , 1×10 1 10-fold gradient mixture in copies / μL; using different concentrations of standards as templates, primers SVA-F, SVA-R, FM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com