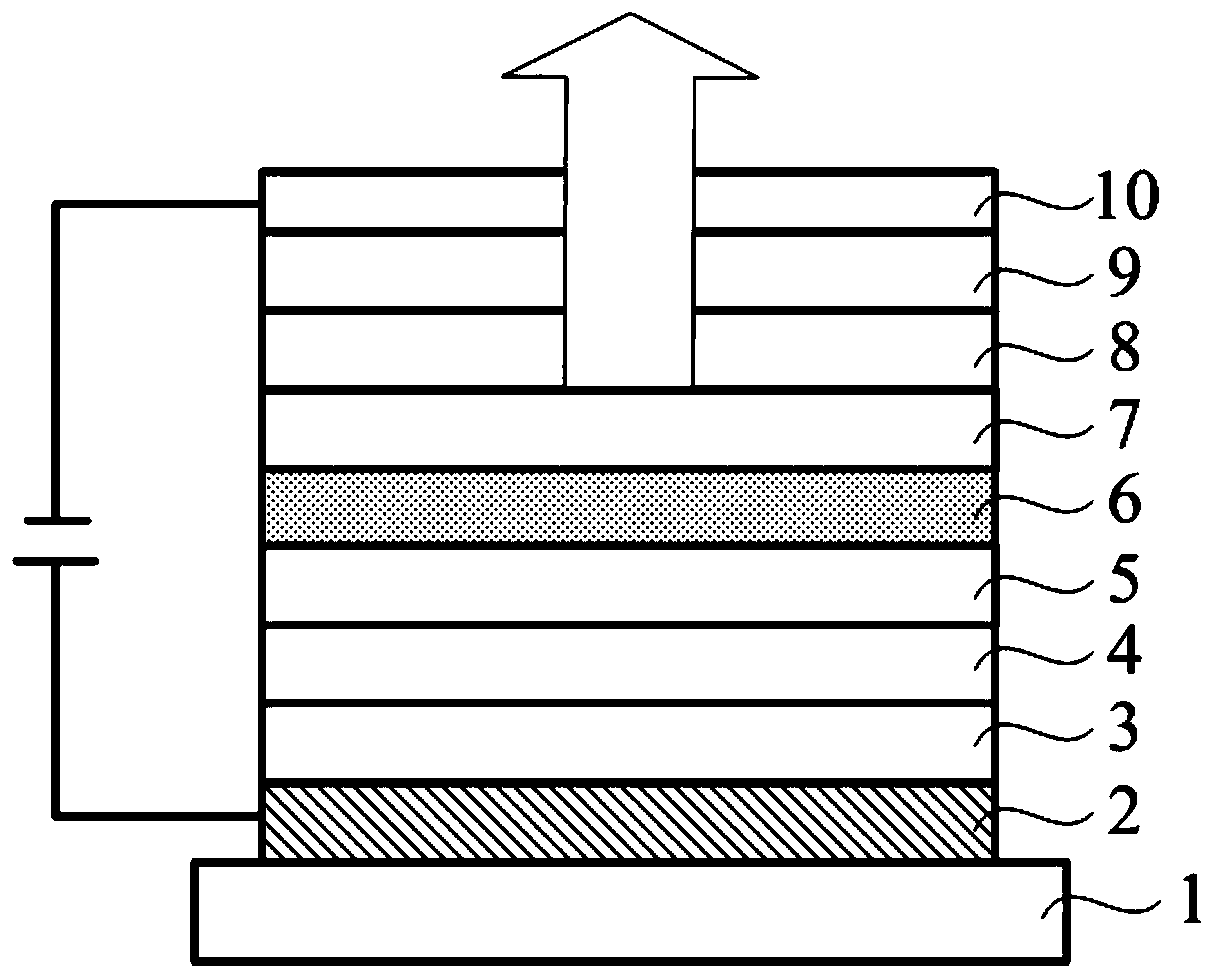
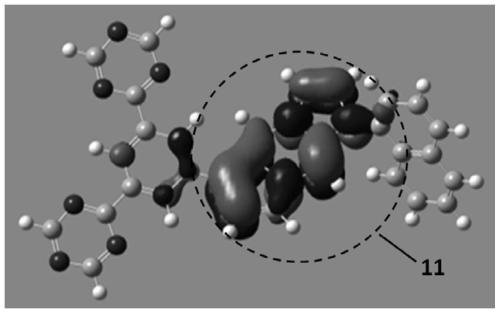
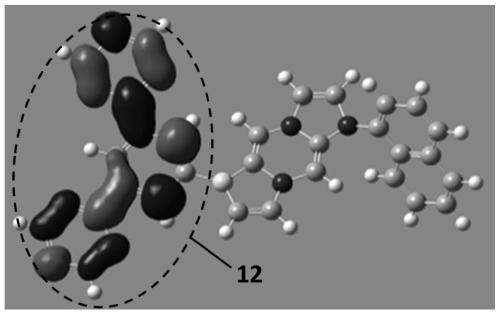
Compounds, organic photoelectric device and electronic device
A technology for organic optoelectronic devices and electronic equipment, applied in the field of organic optoelectronic devices, electronic equipment, and compounds, can solve problems such as performance to be improved, and achieve the effects of reducing device voltage, improving device efficiency, and improving luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0104] This synthesis example provides the preparation method of compound M1, specifically as follows:
[0105]
[0106] (1) Add 13.75g (50mmol) of compound A, 150mL of DCM and 5g (50mmol) of triethylamine in a 250mL three-necked flask in sequence, cool down to 0°C in an ice bath, and slowly drop in 50mL of DCM dissolved in a constant pressure dropping funnel. 10.31g (50mmol) of compound B, after the dropwise addition, was raised to room temperature and stirred, and TCL was used to monitor the reaction progress. After the reaction was complete, it was quenched by adding ice water, extracted with DCM, combined the organic phases, removed the solvent, and recrystallized from Tol / EtOH to obtain product C.
[0107] 1 H NMR (400MHz, Chloroform) δ: 8.02(d, J=7.3Hz, 2H), 7.77(s, 1H), 7.43(d, J=12.0Hz, 3H), 7.29(s, 1H), 7.04(s , 1H), 6.89(s, 1H), 6.21(s, 1H), 5.53(s, 1H), 4.31(s, 1H), 3.68(s, 1H).
[0108] 13 C NMR (100MHz, Chloroform) δ: 169.66(s), 140.32(s), 138.21(s), 136.11...
Synthetic example 2
[0119] This synthesis example provides the preparation method of compound M2, specifically as follows:
[0120]
[0121]
[0122] (1) Add 13.75g (50mmol) of compound A, 150mL of DCM and 5g (50mmol) of triethylamine in a 250mL three-necked flask in sequence, cool down to 0°C in an ice bath, and slowly drop in 50mL of DCM dissolved in a constant pressure dropping funnel. 9.95g (50mmol) of compound B, after the dropwise addition, was stirred at room temperature, and the reaction progress was monitored by TCL. After the reaction was complete, it was quenched by adding ice water, extracted with DCM, combined the organic phases, removed the solvent, and recrystallized from Tol / EtOH to obtain product C.
[0123] 1 H NMR (400MHz, Chloroform) δ: δ7.63(s, 1H), 6.80(s, 1H), 6.60(s, 2H), 6.06(s, 1H), 5.18(s, 1H), 4.65(s, 1H), 3.63(s, 1H), 2.26(s, 3H), 2.13(s, 6H).
[0124] 13 C NMR (100MHz, Chloroform) δ: 169.66(s), 139.71(s), 138.90(s), 137.92(s), 135.96(s), 128.27(s), 126.77(s...
Synthetic example 3
[0135] This synthesis example provides the preparation method of compound M3, specifically as follows:
[0136]
[0137] (1) Add 13.75g (50mmol) of compound A, 150mL of DCM and 5g (50mmol) of triethylamine in a 250mL three-necked flask in sequence, cool down to 0°C in an ice bath, and slowly drop in 50mL of DCM dissolved in a constant pressure dropping funnel. 11.66g (50mmol) of compound B, after the dropwise addition was completed, rose to room temperature and stirred, and TCL monitored the reaction progress. After the reaction was complete, it was quenched by adding ice water, extracted with DCM, combined the organic phases, removed the solvent, and recrystallized from Tol / EtOH to obtain product C.
[0138] 1 H NMR (400MHz, Chloroform) δ: 8.35(s, 2H), 7.75(s, 4H), 7.49(s, 3H), 7.40(d, J=8.0Hz, 6H), 6.85(s, 2H), 6.37 (s, 4H), 6.14(s, 2H), 5.51(s, 2H), 4.71(s, 2H), 3.75(s, 2H).
[0139] 13 C NMR (100MHz, Chloroform) δ: 169.66(s), 140.75(s), 140.35(s), 138.01(s), 135.12(...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com