Fluorene derivative organic electroluminescence device
A technology of fluorene compounds and substituents, applied in the field of organic electroluminescent devices, can solve problems such as uneven molecular weight distribution, unbalanced distribution of electrons and holes, and reduced luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
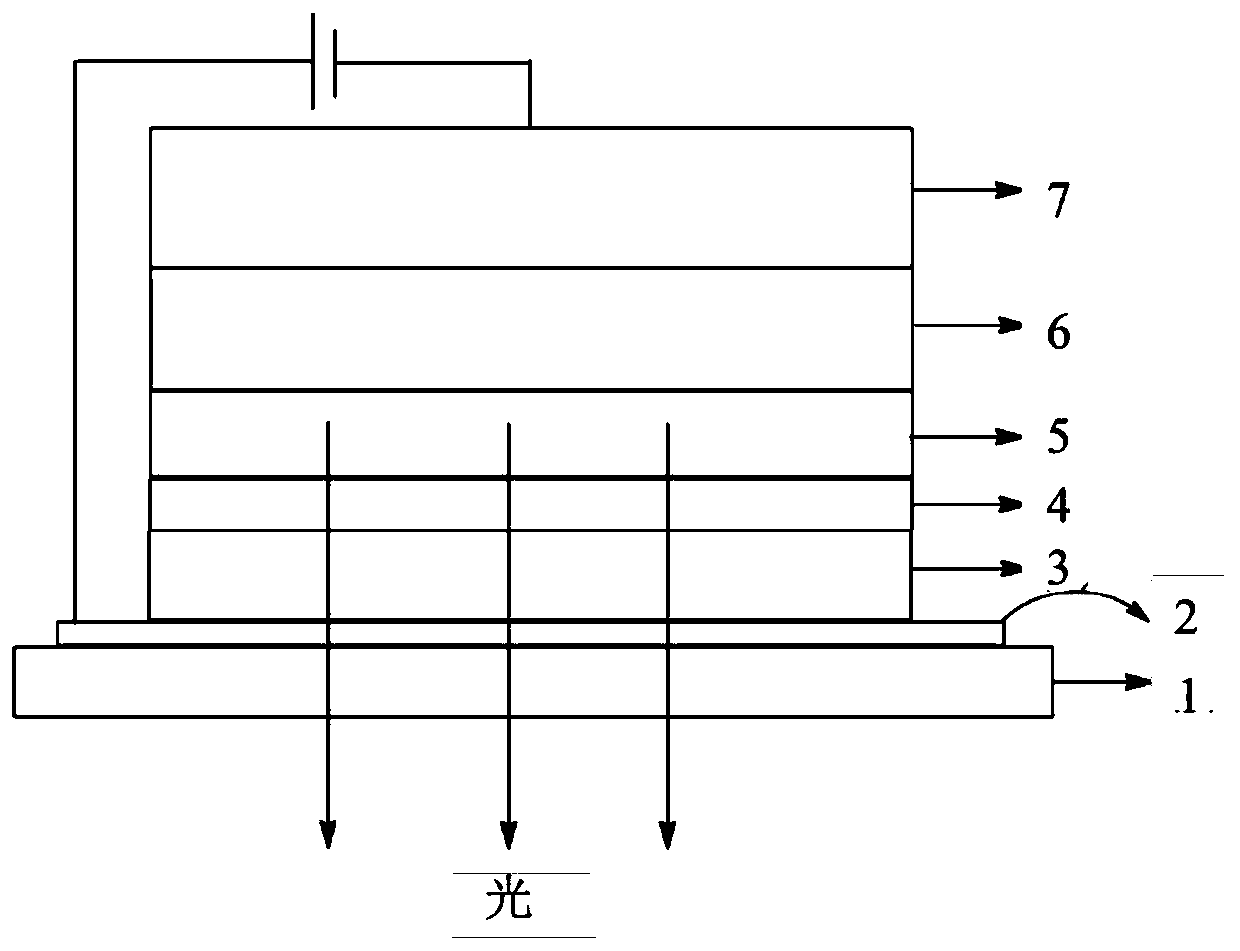
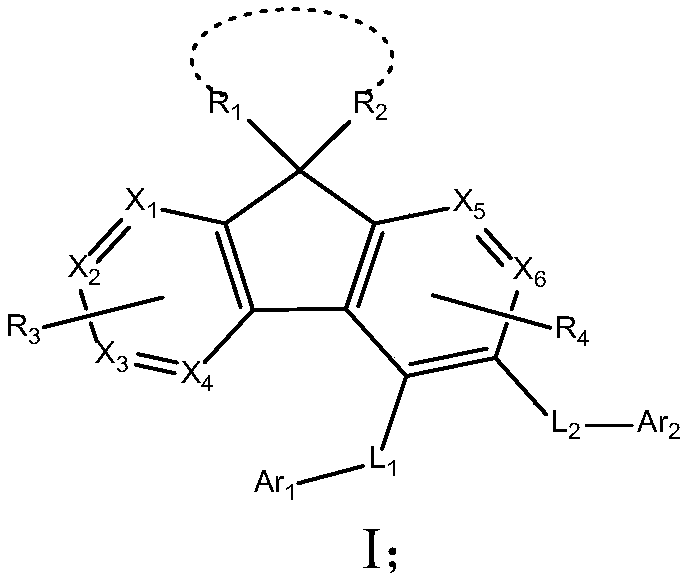
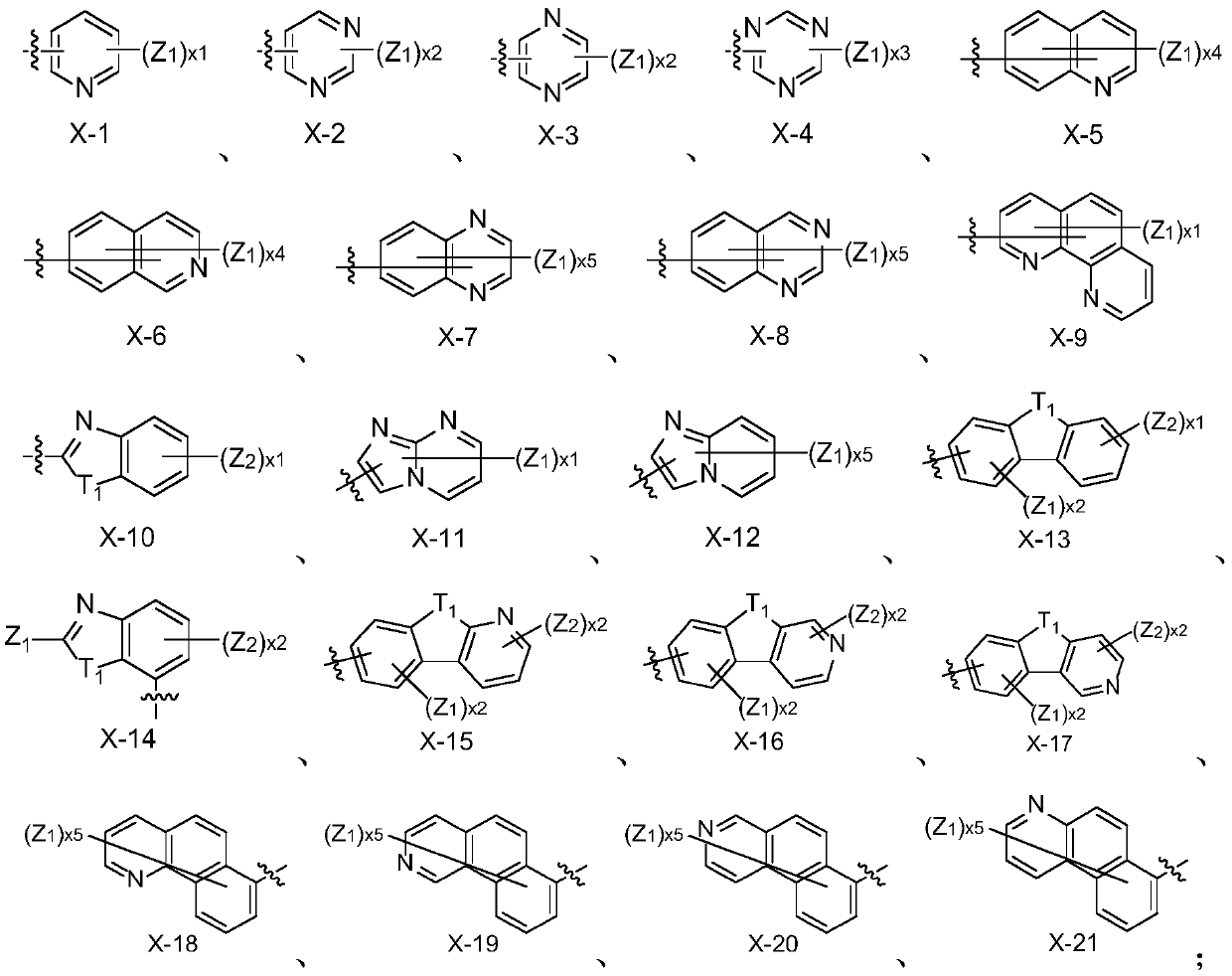
Image
Examples
Embodiment 1
[0072] The preparation method of compound FP31 comprises the following reaction steps:
[0073] Step 1: Preparation of Intermediate Int.-1
[0074]
[0075] The 3-bromo-2-iodophenol of 10.0g (33.4mmol) mixes with 80ml toluene, adds the palladium catalyst Pd ( PPh 3 ) 4 , then add 40ml of ethanol and 40ml of water, heat up and reflux and stir for 12 hours, cool to room temperature, extract with ethyl acetate, dry the organic phase, filter, concentrate the filtrate to dryness under reduced pressure, separate and purify on a silica gel column, and obtain 7.7g of pink solid Int. -1, 92% yield.
[0076] The second step: the preparation of intermediate Int.-2
[0077]
[0078] 10.0g (40.1mmol) of Int.-1 prepared in the first step was dissolved in 150ml of dry THF, under the protection of nitrogen, 4.5g (40.1mmol) of potassium tert-butoxide was added, and the temperature was lowered to -78°C with liquid nitrogen. Add 19.3ml of 2.5M n-butyllithium n-hexane solution dropwise...
Embodiment 2
[0092] Preparation of Compounds FP01~FP30, FP32~FP66 and FP102~FP108
[0093] Referring to the synthesis method of Example 1, the compounds shown in FP01~FP30, FP32~FP66 and FP102~FP108 were prepared, the difference is that according to the different products, different intermediates were selected to replace the intermediate in the sixth step of Synthesis Example 1 The corresponding compounds FP01-FP30, FP32-FP66 and FP102-FP108 were prepared from body L01.
Embodiment 3
[0095] The preparation method of compound FP67 comprises the following reaction steps:
[0096] The first step: the preparation of intermediate Int.-6
[0097]
[0098] 5.0g (23.3mmol) of intermediate Int.-2 and 80ml of tetrahydrofuran were mixed, and 4.2g (19.5mmol) of methyl o-bromobenzoate, 12.4g (46.6mmol) of potassium phosphate hydrate, 0.16g of palladium catalyst Pd were added (PPh 3 ) 4 , then add 20ml of water, heat up to reflux and stir for 12 hours, cool to room temperature, add 100ml of water to dilute, extract with ethyl acetate, dry the organic phase, filter, concentrate the filtrate to dryness under reduced pressure, separate and purify on a silica gel column, and obtain 4.3g of yellow Solid Int.-6, 72% yield.
[0099] The second step: the preparation of intermediate Int.-7
[0100]
[0101] 8.0g (26.3mmol) of the intermediate Int.-6 prepared in the first step was dissolved in 120ml of dry tetrahydrofuran, and under the protection of nitrogen, the tempe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com