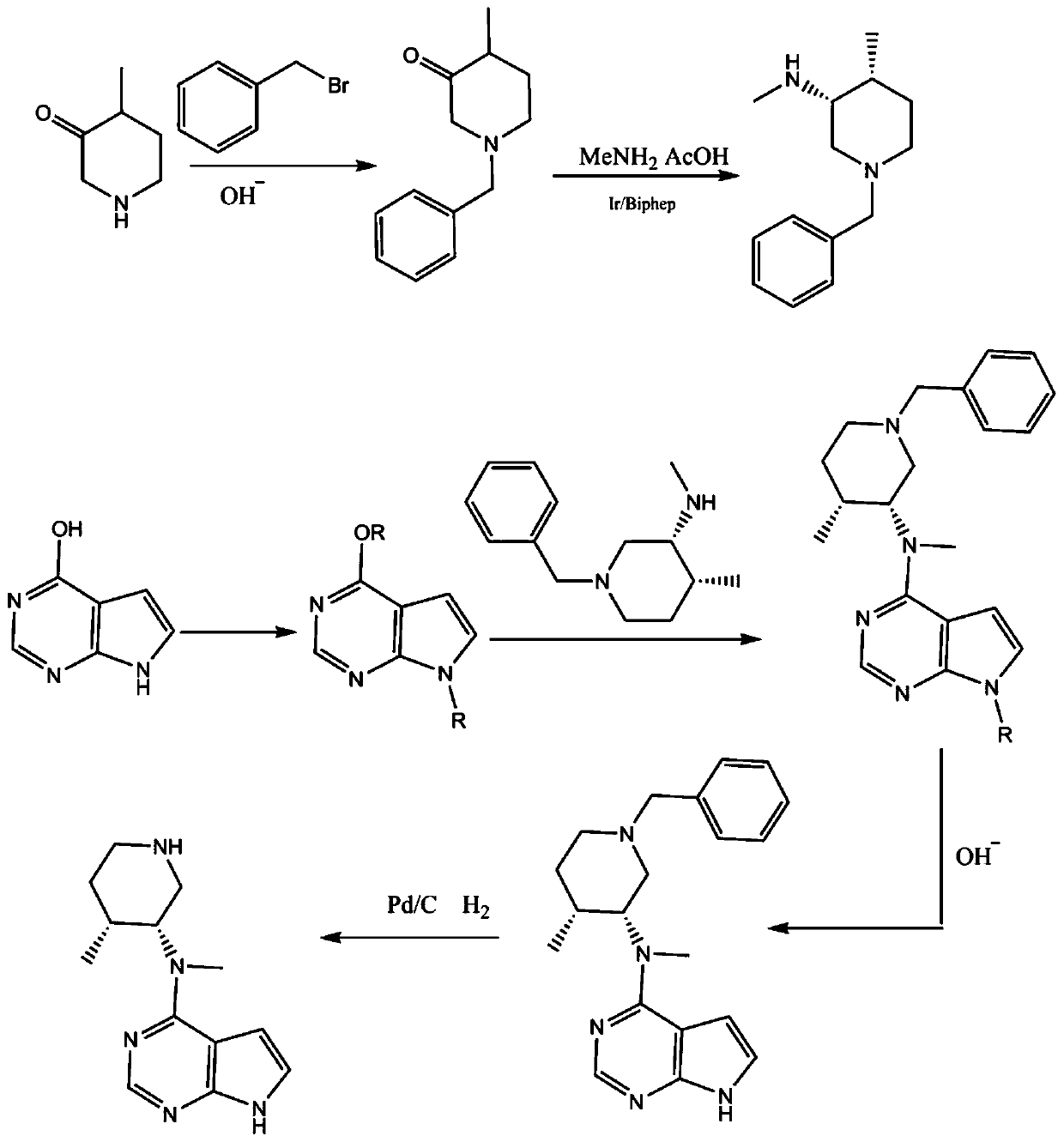
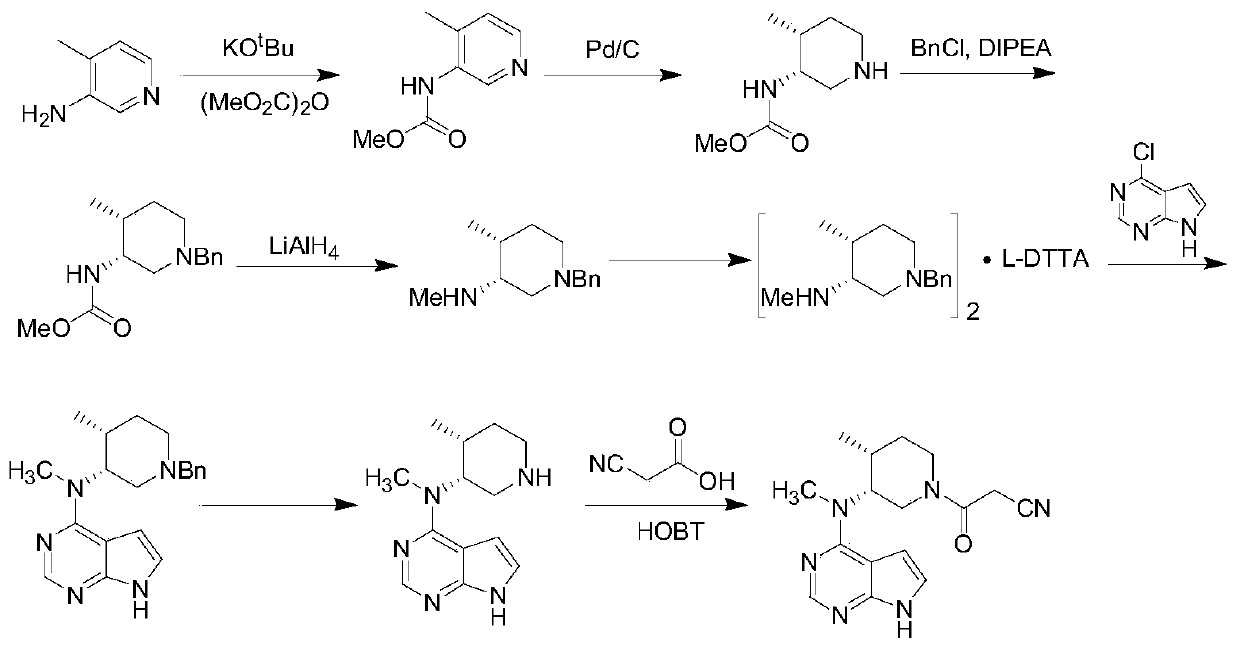
Preparing method of N-methyl-N-(4-methylpiperidine)-3-yl-7H-pyrropyrimidine-4-amine
A technology of pyrrolopyrimidine and methylpiperidine, which is applied in the field of preparation of N-methyl-N--3-yl-7H-pyrrolopyrimidin-4-amine, can solve problems such as danger and reduced yield, Achieve the effect of easy operation, low cost and high chiral purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Step 1: Add 113g of 4-methyl-3-piperidone, 350ml of water and 350ml of methanol into the reaction flask, stir at 0-5°C, add 60g of potassium hydroxide in batches, react for 2 hours, add 171g of bromine dropwise at room temperature Benzyl chloride, dripping, reflux reaction for 6 hours, controlled reaction, after the reaction, the solvent was distilled under reduced pressure, washed with water to obtain 197g of intermediate 1, yield: 97%.
[0028] Step 2: Add 190g of intermediate 1, 362g of monomethylamine aqueous solution, 600ml of methanol and 10g of Ir / Biphep (2,2'-bis(diphenylphosphine)biphenyl) into the reaction flask, feed hydrogen to 1Mpa, and reflux After the reaction, after the reaction, filter, concentrate the filtrate, adjust the pH to 8 with aqueous acetic acid, extract with dichloromethane, concentrate to obtain 163g of intermediate 2, yield: 80%.
[0029] Step 3: Add 135g of 4-hydroxy-6,7-dihydro-5H-pyrrolo[2,3-D]pyrimidine, 650ml of acetonitrile and 345g o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com