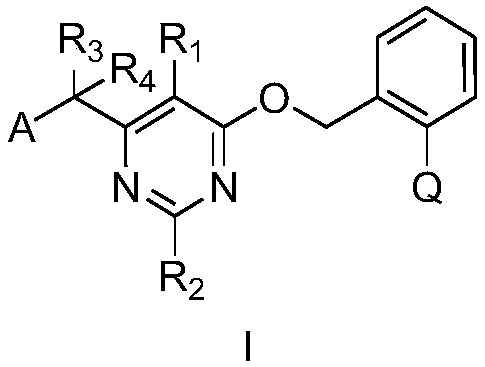
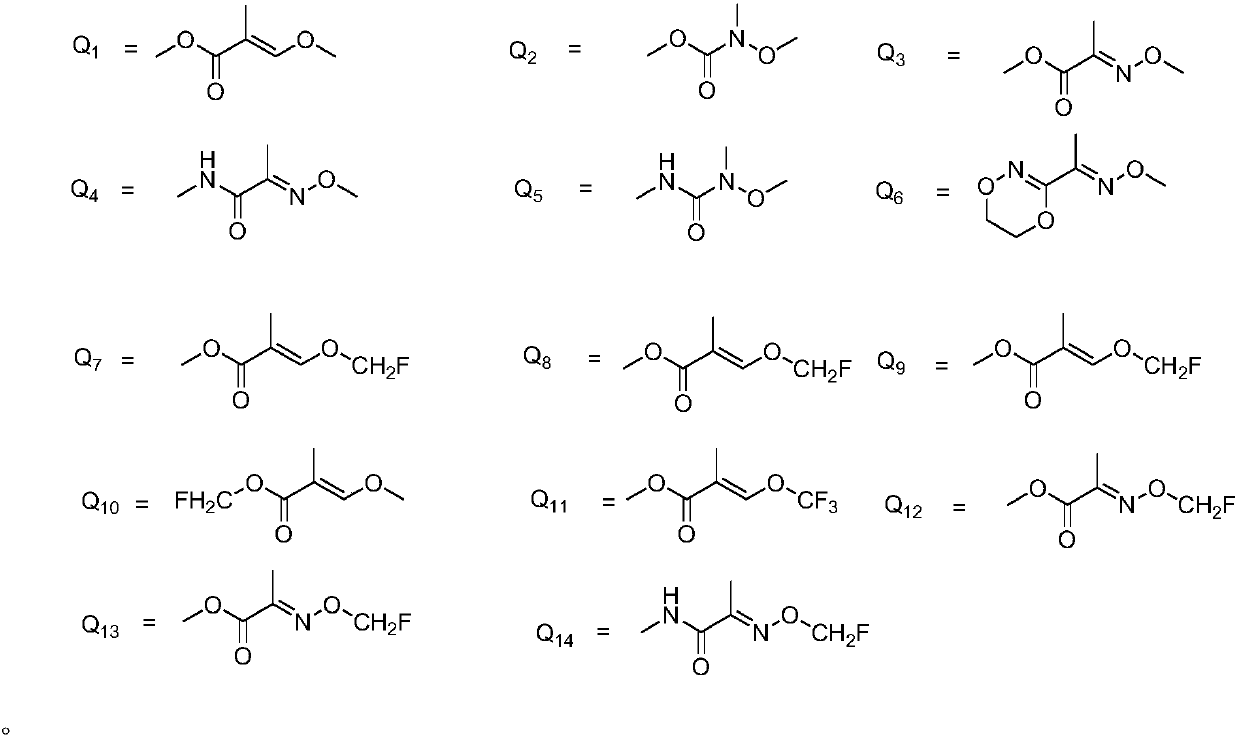
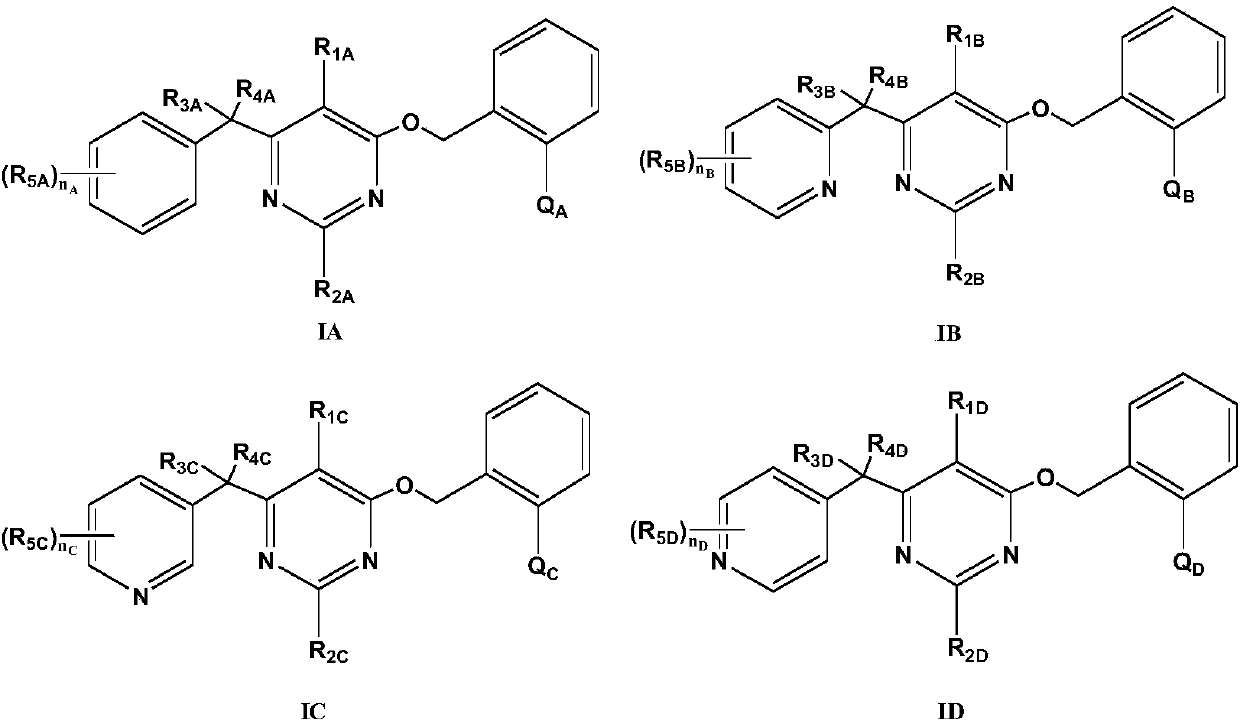
Substituted (hetero) arylmethylene pyrimidine ether compound and preparation method and application thereof
A technology of methyl pyrimidine ether and heteroaryl, which is applied in the field of substituted aryl methylene pyrimidine ether compounds and their preparation, and can solve problems such as unsatisfactory effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0164] Embodiment 1: the synthesis of compound 39
[0165] Dissolve 1.3g (0.005mol) of 1a in 10ml of N,N-dimethylformamide, add 0.83g of potassium carbonate, stir for 0.5h, add 1.26g of 2a in batches, heat up to 80°C after addition, and stir for 8h . After the reaction was detected by TLC, the reaction solution was poured into 50 ml of saturated saline, extracted three times with 100 ml of ethyl acetate, and dried. After precipitation, it was purified by column chromatography to obtain 2.1 g of an oily product.
[0166]
Embodiment 2
[0167] Embodiment 2: the synthesis of compound 15
[0168] Dissolve 1.15g (0.005mol) of 1b in 10ml of N,N-dimethylformamide, add 0.83g of potassium carbonate, stir for 0.5h, add dropwise the DMF solution of 2b (1.4g), and heat up to 80°C after adding , Stir the reaction for 8h. After the reaction was detected by TLC, the reaction solution was poured into 50 ml of saturated saline, extracted three times with 100 ml of ethyl acetate, and dried. After precipitation, it was purified by column chromatography with petroleum ether: ethyl acetate 1000:1-300 eluent to obtain 1.75 g of an oily product.
[0169]
Embodiment 3
[0170] Embodiment 3: the synthesis of compound 207
[0171] Dissolve 2.0g (0.005mol) of 1c in 10ml of N,N-dimethylformamide, add 0.83g of potassium carbonate, stir for 0.5h, add 1.45g of 2c in batches, heat up to 80°C after addition, and stir for 8h . After the reaction was detected by TLC, the reaction solution was poured into 50 ml of saturated saline, extracted three times with 100 ml of ethyl acetate, and dried. After precipitation, it was purified by column chromatography with petroleum ether: ethyl acetate 1000:1-200 eluent to obtain 2.6 g of an oily product.
[0172]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com