Enzyme composition for therapeutic management of muscle soreness
A muscle soreness, therapeutic technology, applied in the field of digestive enzymes, to relieve the symptoms of delayed onset muscle soreness, can solve the problems of limited effectiveness, limited efficacy and inconsistency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Study Design
[0025] Product Description: It is "off-white to milky white powder of multi-enzyme complex". This multi-enzyme complex consists of amylase, protease, lipase, cellulase and lactase. Placebo capsules contained an equivalent weight of maltodextrin. No differences in colour, taste, texture or packaging were detected between the two products. The capsules are sealed in high-density polyethylene bottles of the same appearance as the desiccant. The composition of the multi-enzyme complex is given in Table 1.
[0026] Table 1. Compositional details of the multi-enzyme complex (DigeZyme) formulations
[0027]
[0028] Enzyme units are defined according to Food Chemical Codex (FCC), 5th Edition 2004. The National Academy Press Washington, D.C.
[0029] Ethics approval: Ethics approval was obtained from Sparsh Hospital, Advanced Surgery, #146, Infantry Road, Bangalore (according to gazette notification number F.28-10 / 45-H dated 21 December 1945 ...
Embodiment II
[0037] Example II: Procedure Followed:
[0038] Protocol for Inducing DOMS: Subjects were instructed to ingest study supplements and report to the site after 24 hours for baseline readings. After a 10-hour fast, heart rate and perceived exercise ratings were monitored at rest and every 5 minutes during exercise and recovery for 10 minutes. Participants installed a level motorized treadmill and warmed up at a voluntary pace (pace) for 5 minutes. After a 5-minute warm-up, the treadmill speed was increased until the heart rate reached 80% of the predicted maximum heart rate and was instructed to maintain this pace for 5 minutes. Adjust the treadmill level to 10% at this time and hold for 30 minutes. Subjects then completed a 5-minute active cool-down and a 5-minute seated passive recovery period at a self-selected stride. Subjects were restricted from any other physical activity 24 hours before and 72 hours after the exercise session.
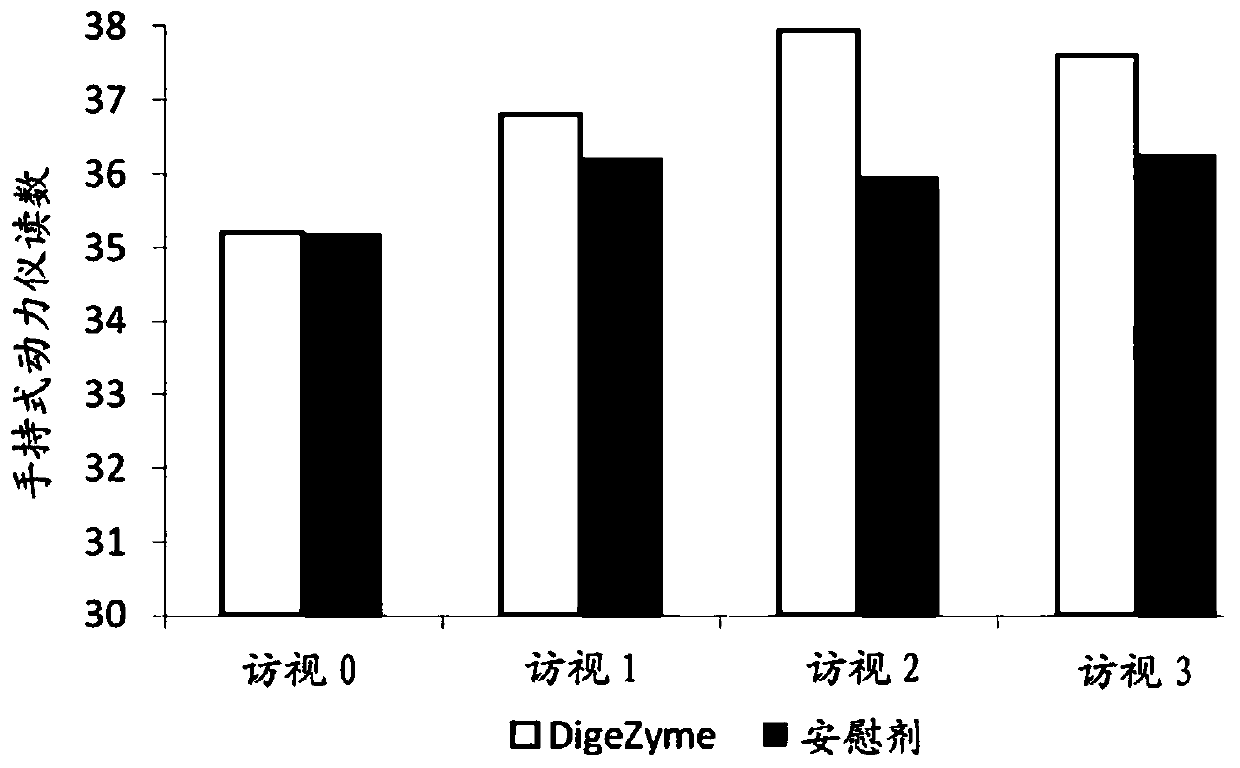
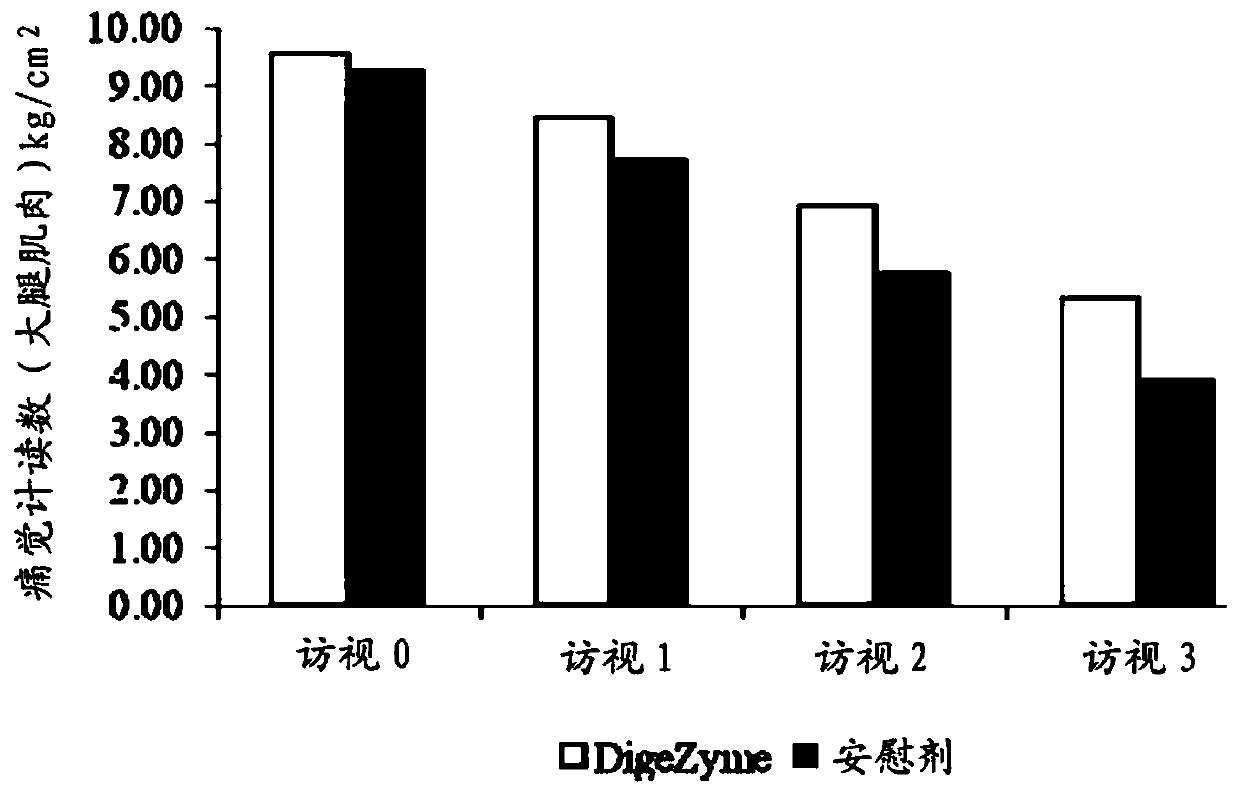
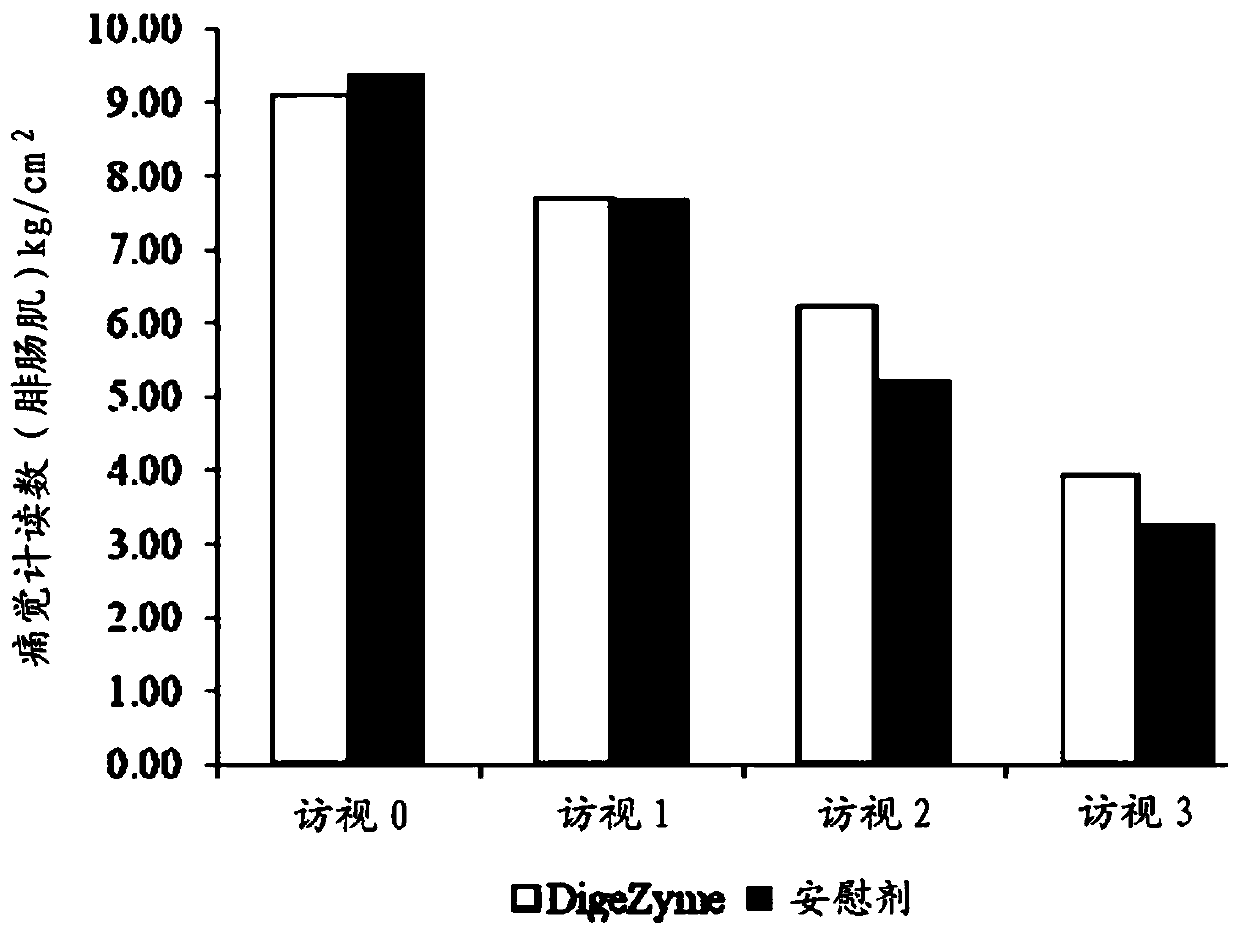
[0039] Muscle soreness was quantified u...
Embodiment III
[0044] Embodiment III: Result:
[0045] Physical Characteristics: The population is generally healthy, with no apparent concomitant disease or drug intake. The screening characteristics of the participants are listed in Table 2.
[0046] Table 2: Subject demographics.
[0047]
[0048] placebo group (n=10) and (n=10) There were no statistically significant differences between subjects in the groups. On the day of screening, the average weight of all enrolled subjects was 59.3±4.64kg; the average height was 163.8±4.99cm, and the average BMI was 22.2±1.50Kg / m 2 .
[0049] Efficacy Assessment: Delayed Onset Muscle Soreness - Quality of life was analyzed throughout the study period as the primary efficacy measure. "p" values indicate statistically significant changes in these symptoms between the placebo and active groups from baseline to final visit. Statistical analysis using analysis of covariance (ANCOVA) showed that the main efficacy parameter was statistically si...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com