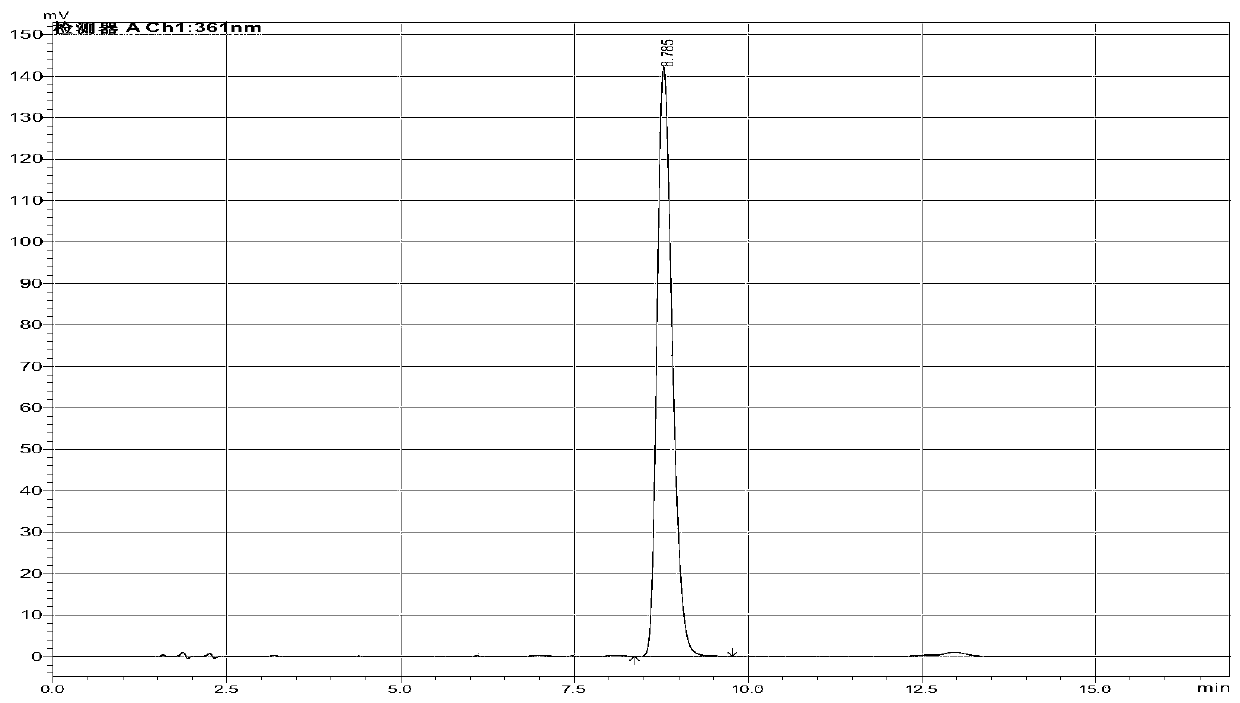
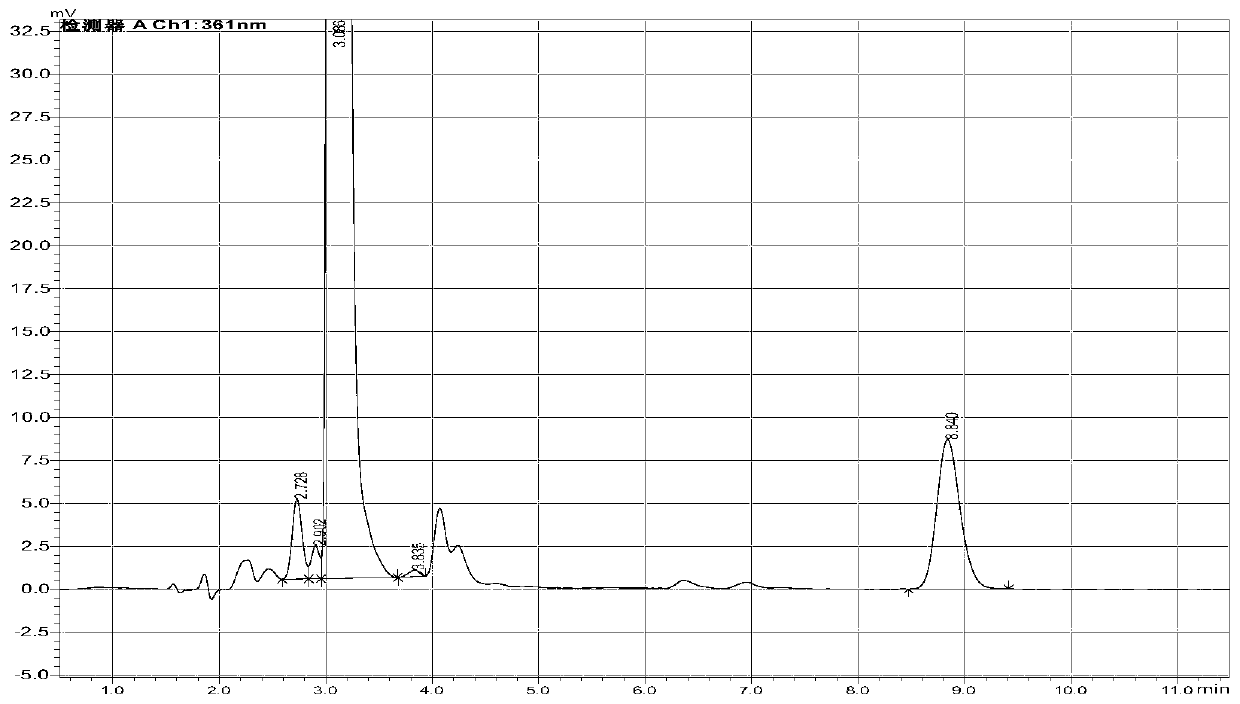
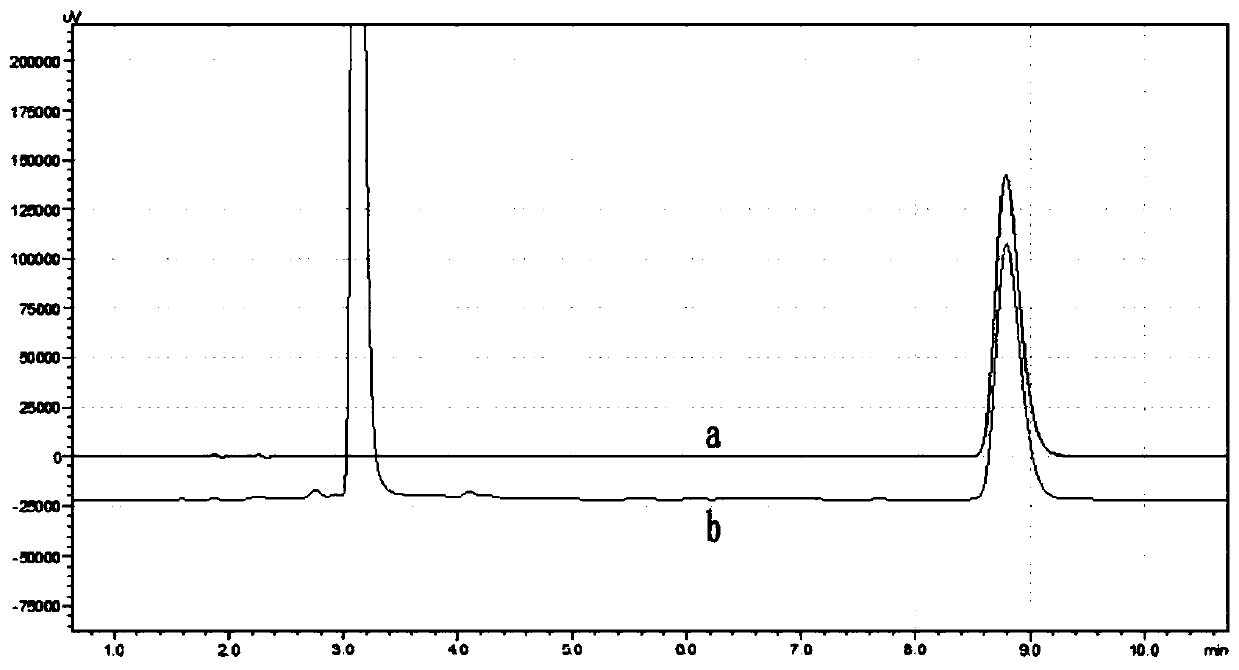
Method of determining cyanide concentration in solution
A cyanide ion and solution technology, which is applied in the field of chemical analysis, can solve the problems of easy decomposition of cyanide, large deviation of measurement results, and easy interference by other ions or colored substances, and achieve the effect of simple operation and safe measurement process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The linear relationship of the method of the present invention is verified and explained.
[0045] (1) Take 100 mL of potassium cyanide standard solution with a calibrated concentration of 87 mg / L for use, and the converted cyanide ion concentration is 34.8 mg / L.
[0046] (2) Weigh 0.40g of hydroxocobalamin acetate, add it to a 1000mL beaker, dissolve and dilute to 1000mL with deionized water.
[0047] (3) Dilute the potassium cyanide standard solution 5, 10, 20, and 50 times respectively as the solution to be tested.
[0048] (4) Take five 100mL volumetric flasks, and add 3 / 4 volume of the hydroxocobalamin acetate solution prepared in step 2 to each volumetric flask, and add 1mL of the solution to be tested to each of the four volumetric flasks, and one volumetric flask Add 1mL of deionized water to it as a blank sample, use deionized water to make up all five volumetric flasks, and bathe in water at 40°C for 60min.
[0049] (5) Dilute the high concentration cyanocob...
Embodiment 2
[0055] The verification of the repeatability of the method of the present invention shows that the concentration of cyanide ions in the sample to be tested is lower than 0.1 mg / L.
[0056] (1) Take 100 mL of potassium cyanide standard solution with a calibrated concentration of 87 mg / L for use, and the converted cyanide ion concentration is 34.8 mg / L.
[0057] (2) Weigh 0.40 g of hydroxocobalamin hydrochloride, dissolve it with deionized water, and dilute to 10 mL to prepare a concentrated solution of hydroxocobalamin hydrochloride.
[0058] (3) Dilute the potassium cyanide standard solution by 1000 times as the solution to be tested.
[0059] (4) Take five 100mL volumetric flasks and rinse them with the solution to be tested. Add 1mL hydroxocobalamin hydrochloride concentrated solution to 4 volumetric flasks, and add 1mL deionized water to the other 1 volumetric flask. Use all 5 volumetric flasks The solution to be tested was fixed to volume and placed in a water bath at 40°...
Embodiment 3
[0065] The cyanide ion concentration of the sample to be tested is higher than 0.1 mg / L, and the repeatability of the determination method of the present invention is verified.
[0066] (1) Take 100 mL of potassium cyanide standard solution with a calibrated concentration of 87 mg / L for use, and the converted cyanide ion concentration is 34.8 mg / L.
[0067] (2) Weigh 0.40g of hydroxocobalamin, put it into a 1000mL beaker, dissolve and dilute to 1000mL with deionized water.
[0068] (3) Dilute the potassium cyanide standard solution 10 times as the solution to be tested.
[0069] (4) Take five 100mL volumetric flasks and add 3 / 4 volume of hydroxocobalamin solution to the volumetric flasks. Add 1mL of the solution to be tested to four of the volumetric flasks, and add 1mL of deionized water to the other one. Dilute to volume with deionized water, and bathe in 40°C water bath for 60min.
[0070] (5) Dilute the high concentration cyanocobalamin standard solution (100.4mg / L) 25 t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com