Oxygen vacancy-rich ferric oxide nitrogen fixation catalyst based on reducing ionic liquid, preparation method and its electrocatalytic nitrogen fixation application
A ferric oxide, ionic liquid technology, applied in catalyst activation/preparation, metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, etc., can solve the complex preparation operation, ammonia yield and Faradaic efficiency Can not meet the requirements of industrial production, complex preparation methods and other problems, to achieve the effects of cheap and easily available raw materials, excellent nitrogen reduction catalytic performance, and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]A method for preparing an oxygen-vacancy-rich ferric oxide nitrogen-fixing catalyst based on reducing ionic liquids, comprising the steps of:
[0038] In an ice-water bath (0°C), add formic acid drop by drop to octylamine (the molar ratio of formic acid and octylamine is 1:1), stir while adding dropwise, after the dropwise addition, stir and mix evenly until no white smoke, a white solid was obtained, that is, a reducing ionic liquid (OAF) was successfully synthesized.
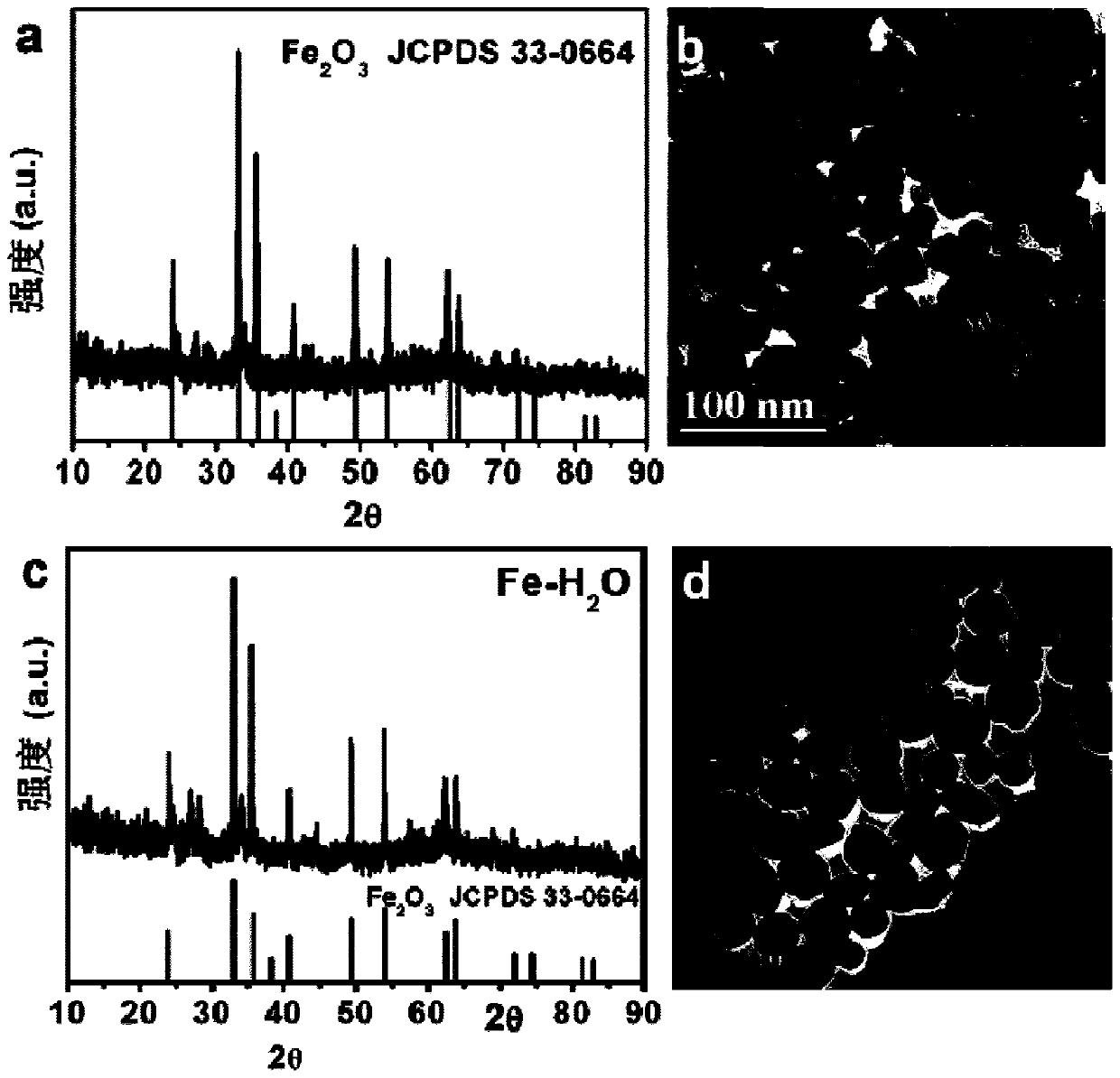
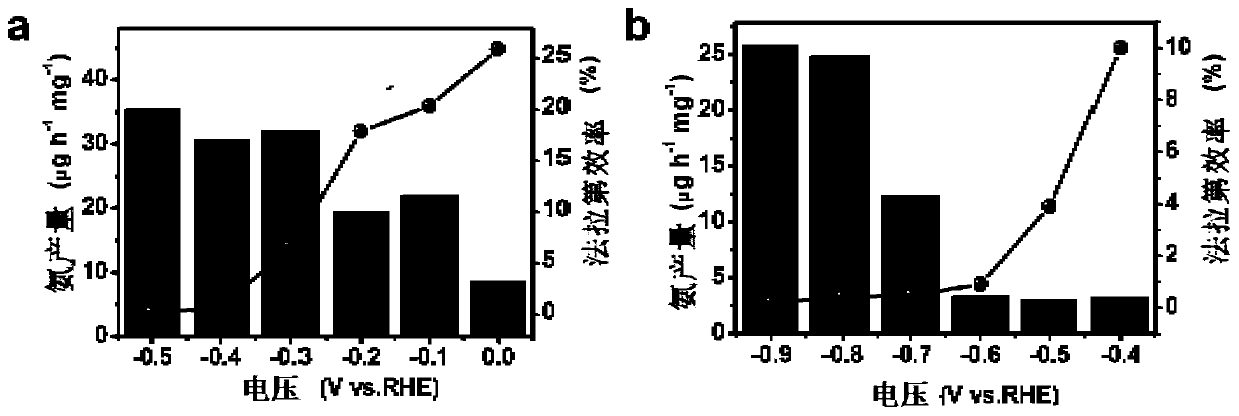
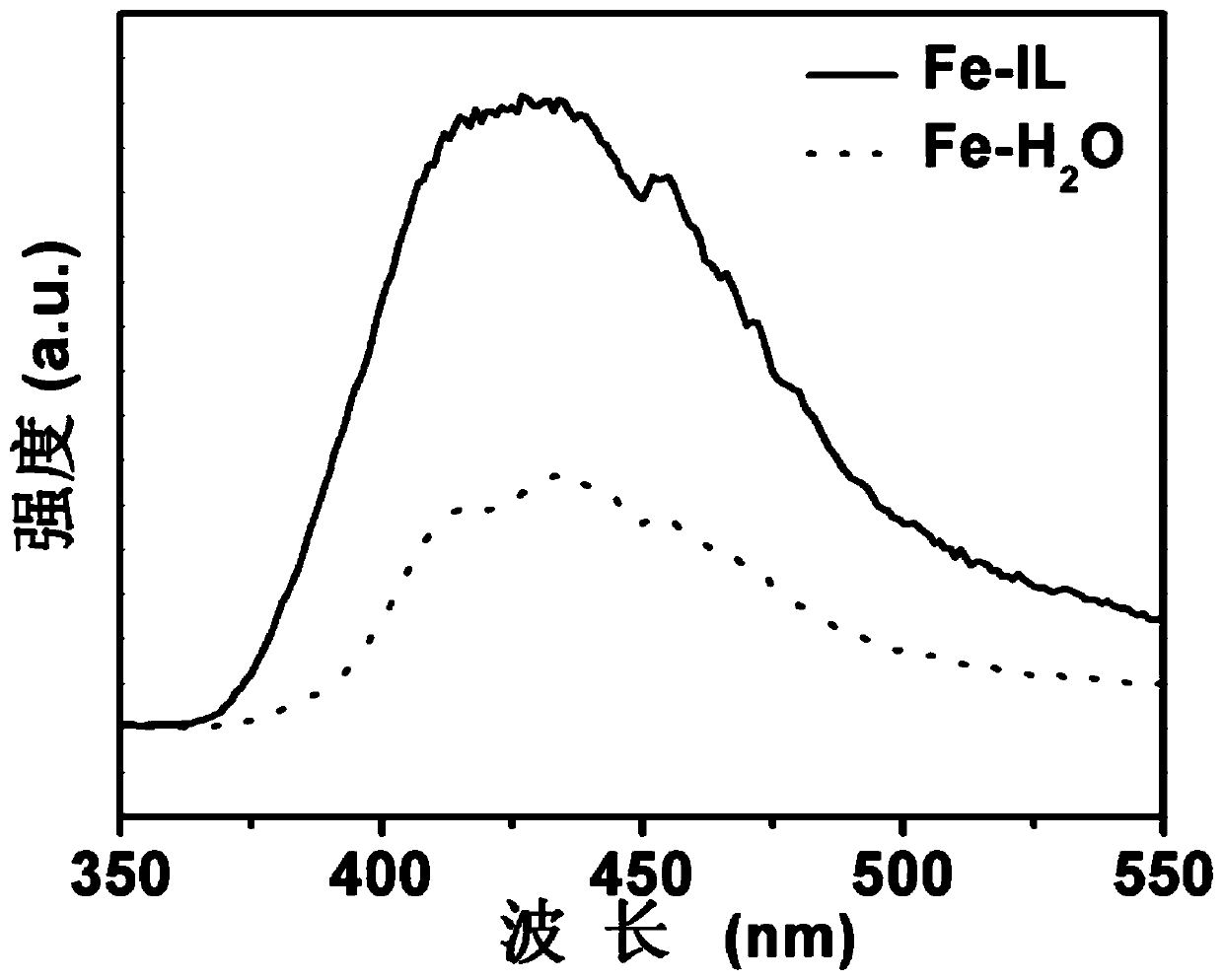
[0039] 30mg (1.1×10 -4 mol) FeCl 3 ·6H 2 O and 4g (0.023mol) of OAF were thoroughly mixed evenly, then placed in a closed reactor, and subjected to ionothermal reaction at 180°C for 12 hours; after the reaction was completed, cooled to room temperature, centrifuged, washed 4 times with absolute ethanol and deionized water, and then The product was put into a vacuum drying oven and dried at room temperature for 12 hours to obtain a brown-red solid powder, which is a nitrogen fixation catalyst rich in o...
Embodiment 2
[0052] A method for preparing an oxygen-vacancy-rich ferric oxide nitrogen-fixing catalyst based on reducing ionic liquids, comprising the steps of:
[0053] In an ice-water bath (0°C), add formic acid drop by drop to octylamine (the molar ratio of formic acid and octylamine is 1:1), stir while adding dropwise, after the dropwise addition, stir and mix evenly until no white smoke, a white solid was obtained, that is, a reducing ionic liquid (OAF) was successfully synthesized.
[0054] 30mg (1.1×10 -4 mol) FeCl 3 ·6H 2 O and 2g (0.012mol) of OAF were thoroughly mixed evenly, then placed in a closed reactor, and subjected to ionothermal reaction at 140°C for 15 hours; after the reaction was completed, cooled to room temperature, centrifuged, washed 4 times with absolute ethanol and deionized water, and then The product was put into a vacuum oven and dried at room temperature for 10 h to obtain a brown-red solid powder.
Embodiment 3
[0056] A method for preparing an oxygen-vacancy-rich ferric oxide nitrogen-fixing catalyst based on reducing ionic liquids, comprising the steps of:
[0057] In an ice-water bath (0°C), add formic acid drop by drop to octylamine (the molar ratio of formic acid and octylamine is 1:1), stir while adding dropwise, after the dropwise addition, stir and mix evenly until no white smoke, a white solid was obtained, that is, a reducing ionic liquid (OAF) was successfully synthesized.
[0058] 30mg (1.1×10 -4 mol) FeCl 3 ·6H 2 O and 6g (0.035mol) of OAF were thoroughly mixed evenly, then placed in a closed reactor, and subjected to ionothermal reaction at 220°C for 8 hours; after the reaction was completed, cooled to room temperature, centrifuged, washed 4 times with absolute ethanol and deionized water, and then The product was put into a vacuum oven and dried at room temperature for 15 hours to obtain a brown-red solid powder.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com