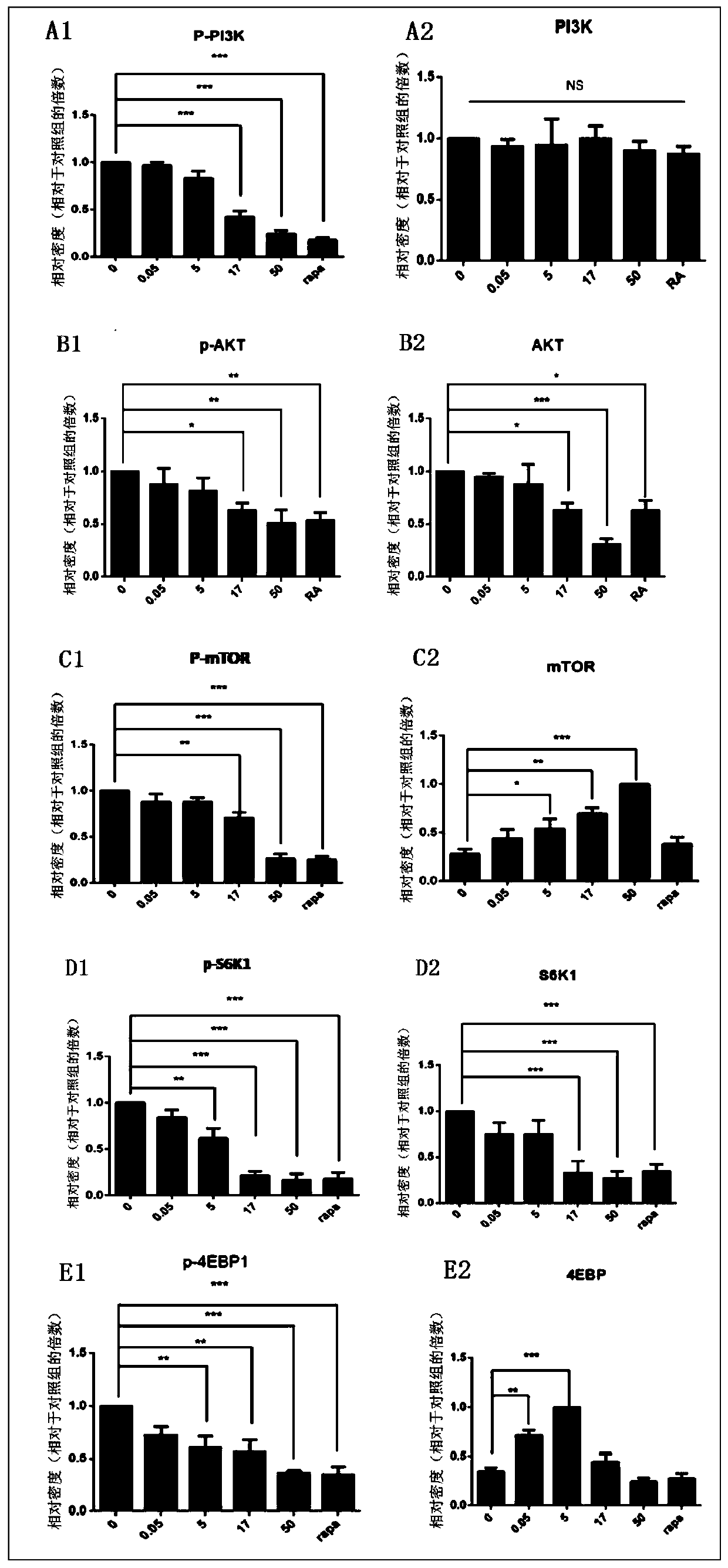
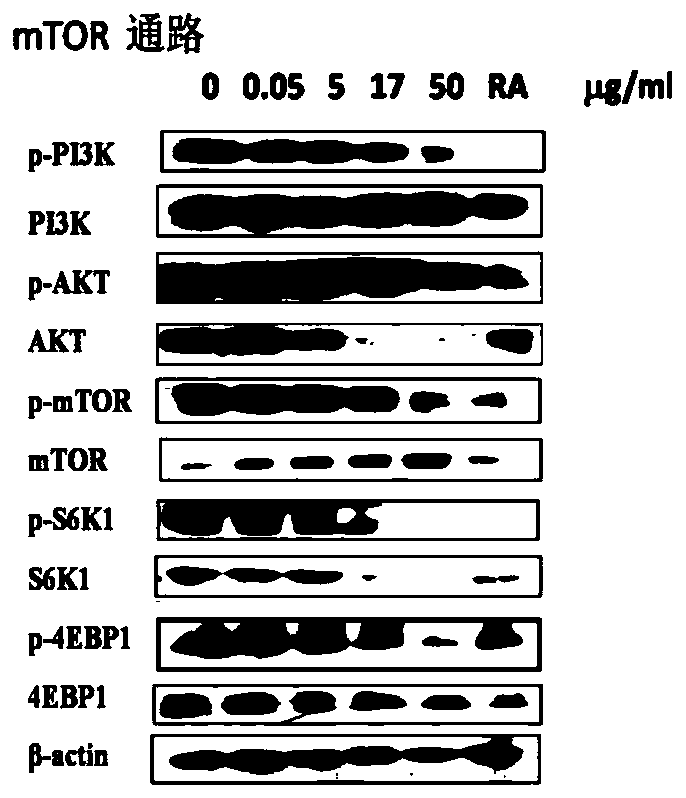
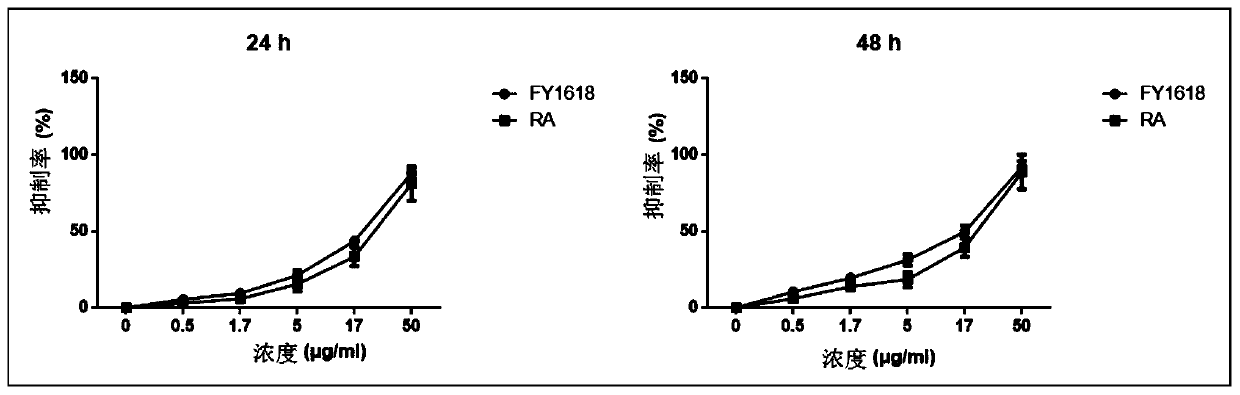
MTOR inhibitor and pharmaceutical composition, and application thereof
A technology of inhibitors and compositions, applied in the field of pharmaceutical compositions, mTOR inhibitors, capable of solving problems such as complex interactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] Embodiment 1, isovalerylspiramycin I or isovalerylspiramycin II or isovalerylspiramycin III tablet
[0108] Specification: 200mg / 350mg
[0109] Tablet prescription:
[0110]
[0111] Coating Solution Prescription:
[0112]
[0113] Preparation Process:
[0114] Preparation of the tablet core: the main drug and auxiliary materials are respectively passed through a 100-mesh sieve, and the prescription amount of isovalerylspiramycin I or isovalerylspiramycin II or isovalerylspiramycin III, microcrystalline cellulose and 1 / 2 The prescription amount of sodium starch glycolate is mixed evenly, then add 5% povidone K 30 The soft material made from aqueous solution is granulated with a 18 mesh sieve, and the wet granules are dried at 60°C under ventilated conditions for 2 hours; after drying, the granules are sieved with an 18 mesh sieve, and then add 1 / 2 of the prescription amount of sodium carboxymethyl starch and stearic acid After the magnesium was evenly mixed, t...
Embodiment 2
[0117] Embodiment 2, isovalerylspiramycin I or isovalerylspiramycin II or isovalerylspiramycin III plain sheet (calculated by 10000 pieces)
[0118] prescription:
[0119] Isovalerylspiramycin I or Isovalerylspiramycin II or Isovalerylspiramycin III powder 1000g
[0120] Low-substituted hydroxypropyl cellulose (5%) 92.5g
[0121] Sodium carboxymethyl starch (3%) 55.5g
[0122] Magnesium stearate (1%) 18.5g
[0123] Total weight of starch - weight of other raw and auxiliary materials
[0124] Total weight 1850g
[0125] Preparation process: Weigh an appropriate amount of starch, dilute to 15% concentration, heat to a paste, and make an adhesive; the main ingredient isovalerylspiramycin I or isovalerylspiramycin II or isovalerylspiramycin III , auxiliary material starch, low-substituted hydroxypropyl cellulose, sodium carboxymethyl starch, and magnesium stearate were passed through a 100-mesh sieve respectively, and the required main materials and auxiliary materials were w...
Embodiment 3
[0126] Embodiment 3, isovalerylspiramycin I or isovalerylspiramycin II or isovalerylspiramycin III capsules (calculated by 10000 grains)
[0127] prescription:
[0128] Isovalerylspiramycin I or Isovalerylspiramycin II or Isovalerylspiramycin III powder 1000g
[0129] Starch 1080-isovalerylspiramycin I powder weight
[0130] Medicinal Capsule No. 3 1000 capsules
[0131] Liquid paraffin 50ml
[0132] Preparation process: Weigh the main material isovalerylspiramycin I or isovalerylspiramycin II or isovalerylspiramycin III, and the excipient medical starch according to the process formula, put them into a mixer and mix thoroughly 1.5-2 hours; the data obtained by sampling and testing the content should be basically consistent with the theoretical data (the weight of each capsule is about 0.105g), and the qualified medicinal No. According to the operation requirements of the machine, fill them into the feeder respectively, and conduct a difference test (within ±10%, <0.3g) fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com