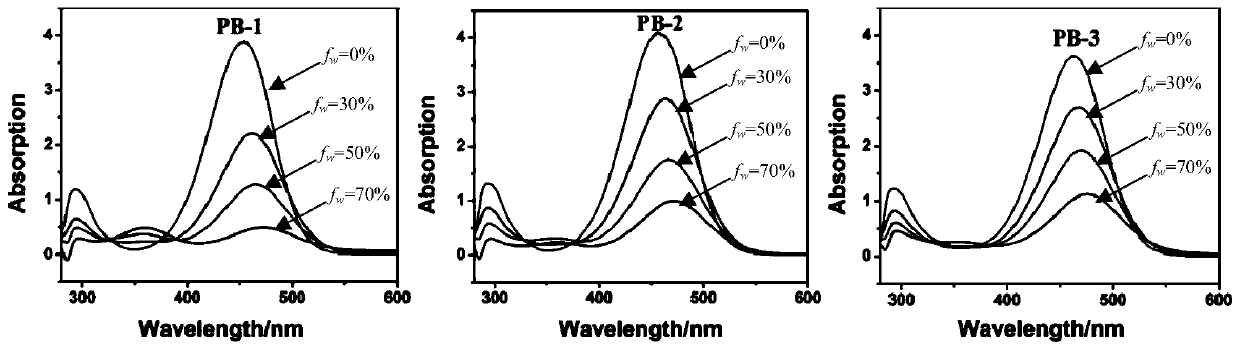
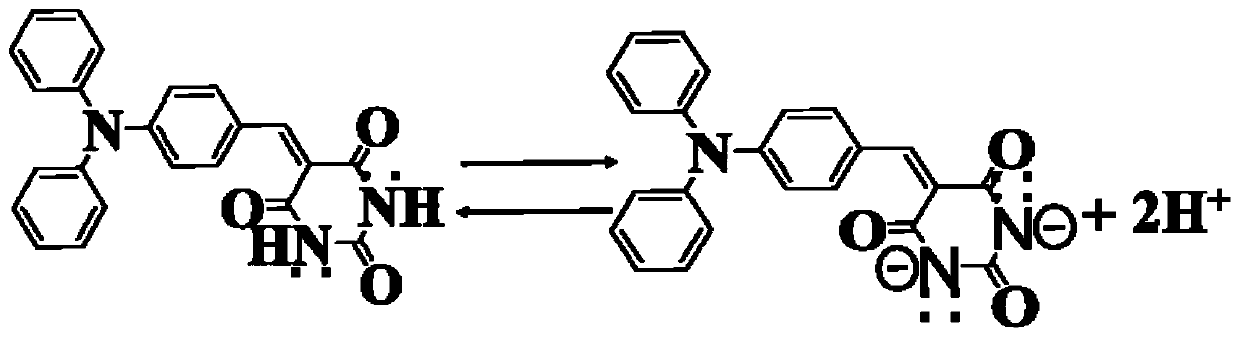
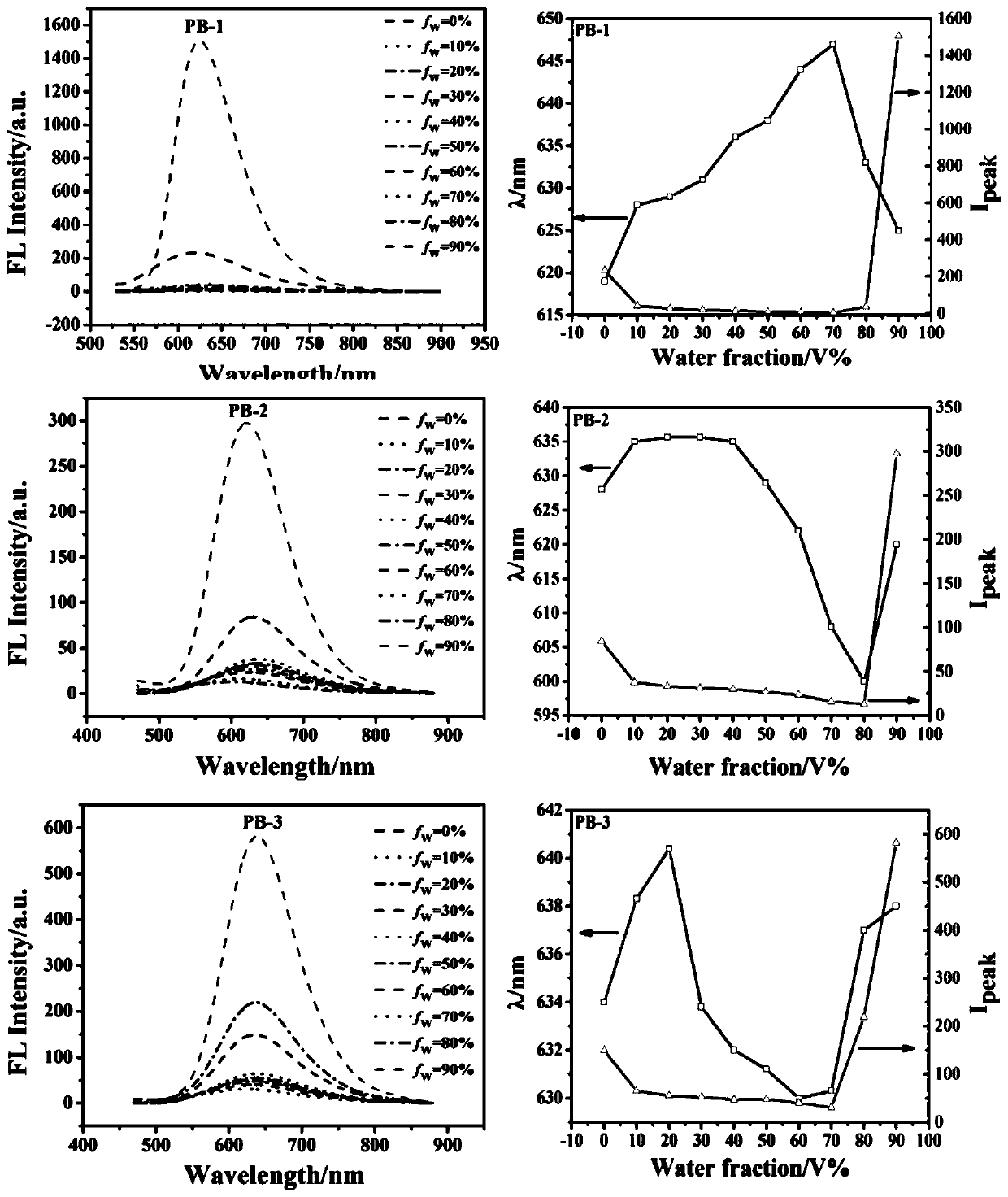
Barbituric acid derivative as well as preparation method and application thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] The synthesis of 4-(diphenylamino)-benzaldehyde, the route is as follows:
[0058]
[0059] At 0°C, 2 mL of anhydrous (N,N-dimethylformamide) DMF (25.7 mmol) was added to 5 mL of phosphorus oxychloride (POCl 3 ) (54mmol) and held for 15 minutes, then triphenylamine (1 g, 4mmol) was added with stirring. The mixture was heated to 45°C for 2.5 hours. After cooling, the clear red solution was dropped into ice water. The resulting mixture was filtered, washed and redissolved in dichloromethane. The solution was washed with water (150 mL) and dried over magnesium sulfate. After evaporation of the solvent, column chromatography gave the pure product, ie, 4-(dianilino)-benzaldehyde. Yield: 0.78 g (80%). 1 H NMR (400MHz, CDCI 3 ):δ9.82(s,1H),7.74–7.61(m,2H),7.35(t,J=7.7Hz,4H),7.22–7.13(m,6H),7.07–6.96(m,2H).
Embodiment 2
[0061] The synthesis of 1,3-diphenylbarbituric acid is as follows:
[0062]
[0063] Malonyl chloride (33.3 mg, 2 mmol) was added to a solution containing 6 ml of CHCl 3 N,N-diphenylurea (424.5mg, 2mmol), and the mixture was heated to 80°C for 4 hours. Then the reaction mixture was extracted with dichloromethane, and the 2 SO 4 Dry on top. After filtration, the filtrate was concentrated under reduced pressure and further purified by column chromatography to obtain 1,3-diphenylbarbituric acid. Yield: 343.1 mg (75%). 1 H NMR (400MHz, DMSO-d6): δ7.45(d, J=7.5Hz, 3H), 7.41(d, J=7.5Hz, 1H), 7.28(d, J=7.4Hz, 3H), 4.01( s, 1H).
Embodiment 3
[0065] The synthesis of 5-(4-diphenylaminostyrene)-barbituric acid (PB-1) is as follows:
[0066]
[0067] A mixture of 4-(dianilino)-benzaldehyde (0.447g, 3mmol) and barbituric acid (0.384g, 3mmol) in (10ml) ethanol was refluxed for 4 hours. After filtration, the filtrate was concentrated under reduced pressure and further purified by column chromatography (ethyl acetate:petroleum ether=2:1). Yield: (0.75 g) 90%. 1 H NMR (400MHz, CDCl3) δ (ppm): 8.42 (s, 1H), 8.31 (d, J = 8.9Hz, 2H), 7.97 (s, 1H), 7.80 (s, 1H), 7.40 (t, J =7.7Hz,4H),7.24(t,J=6.3Hz,6H),6.94(d,J=8.9Hz,2H).FT-IR(KBr,cm -1 ):3217(N-H), 3061(=C-H), 1672(C=O).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com