Valsartan impurity and preparation method thereof
A kind of technology of valsartan and impurity, applied in N-methyl)-N-nitroso-L-valine and preparation field thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
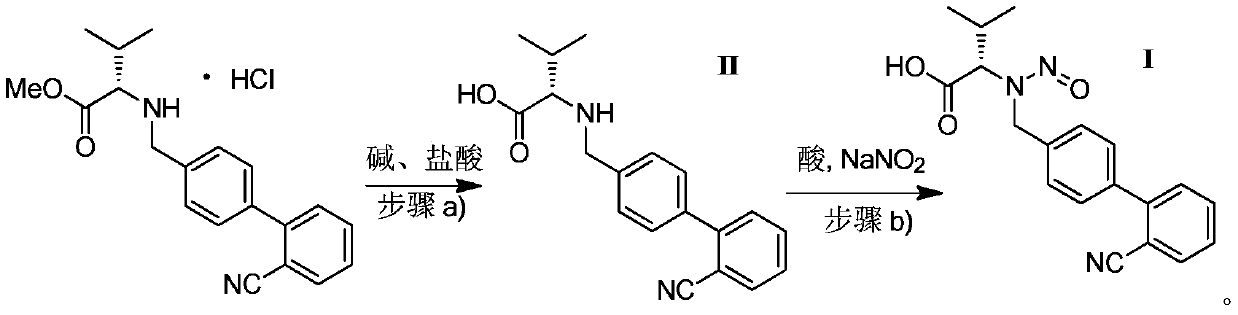
[0021] Synthesis of N-((2'-cyano-[1,1'-biphenyl]-4-yl)methyl)-L-valine (compound of formula II):
[0022] Into a 1000mL three-neck flask, add 35.8g (0.1mol) N-((2'-cyano-[1,1'-biphenyl]-4-yl)methyl)-L-valine methyl ester successively Hydrochloride, 300g of methanol, stirred for 10 minutes, added a solution prepared by 40g (1.0mol) NaOH and 200mL of water, kept at 30-40°C, and stirred for 5h. Hydrochloric acid was added dropwise to pH = 5-6, and stirring was continued for 15 minutes after the dropwise addition was completed. The filtrate was filtered off and the filter cake was retained. The filter cake was washed with 100 g of water. Dry in a vacuum oven to obtain the compound of formula II, 28.9 g of white solid, HPLC purity: 98%, yield 94%.
Embodiment 2
[0024] Synthesis of N-((2'-cyano-[1,1'-biphenyl]-4-yl)methyl)-L-valine (compound of formula II):
[0025] Into a 1000mL three-necked flask, add 35.8g (0.04mol) N-((2'-cyano-[1,1'-biphenyl]-4-yl)methyl)-L-valine methyl ester successively Hydrochloride, 200g of ethanol, stir for 10 minutes, add a solution prepared by 44.8g (0.8mol) KOH and 200mL of water, keep warm at 40-45°C, and react for 20h. Hydrochloric acid was added dropwise to pH = 5-6, and stirring was continued for 15 minutes after the dropwise addition was completed. The filtrate was filtered off and the filter cake was retained. The filter cake was washed with 100 g of water. Dry in a vacuum oven to obtain the compound of formula II, 28.3 g of white solid, HPLC purity: 99%, yield 92%.
Embodiment 3
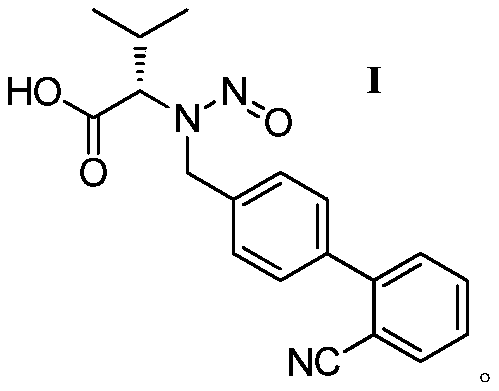
[0027] Synthesis of N-((2'-cyano-[1,1'-biphenyl]-4-yl)methyl)-N-nitroso-L-valine (formula I valsartan impurity):
[0028] In the there-necked flask of 500mL, add 30.8g (0.1mol) formula II compound successively, the methanol of 150g, the dichloromethane of 150g, 14.4g (0.15mol) methanesulfonic acid, after stirring for 10 minutes, slowly dropwise add 10.3g ( 0.15mol) NaNO 2 Prepare the solution with 20mL of water, react for 1-2 hours, filter, and dry the filter cake to obtain the impurity of formula I valsartan, 26.3g of off-white powder, HPLC purity: 95%, yield 78%. MS-ESI(m / z):[M+H]+338.4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com