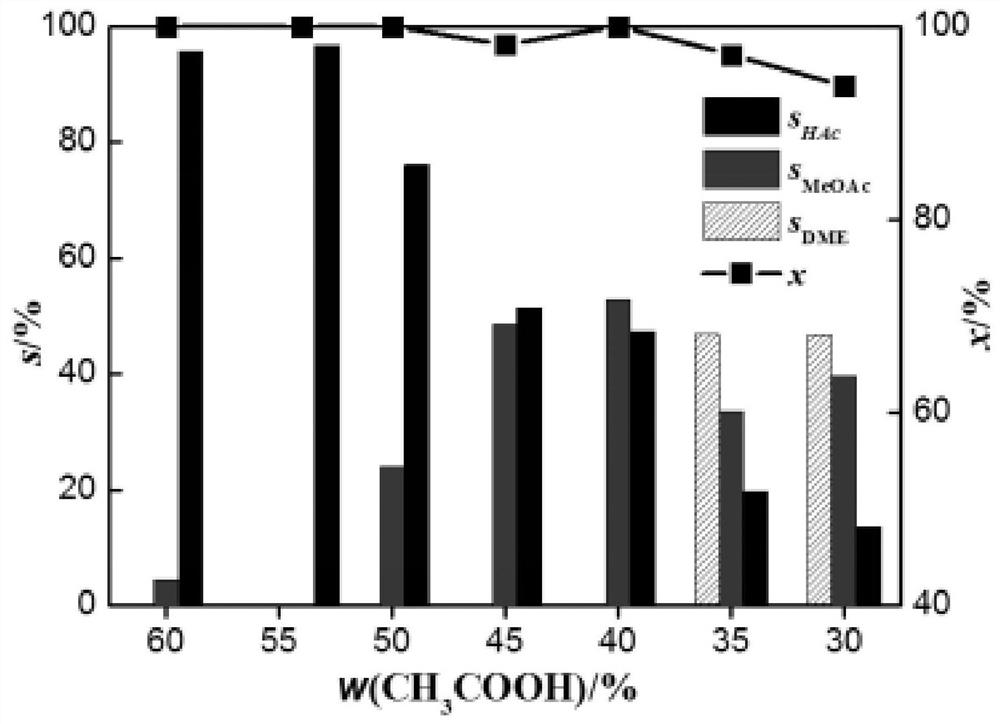
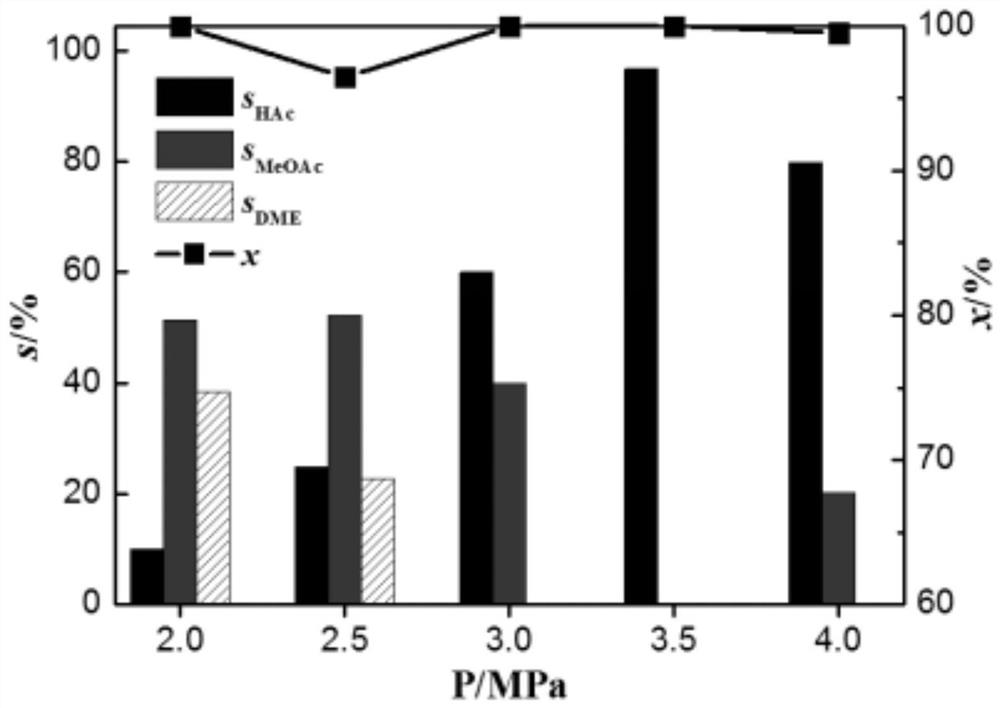
Pyridyl rhodium catalyst and its preparation method and application
A technology of pyridyl rhodium and catalyst, which is applied in the field of pyridyl rhodium catalyst and its preparation, can solve the problems of price change, lower conversion rate, and increase cost, and achieve the effects of maintaining activity, high conversion rate, and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
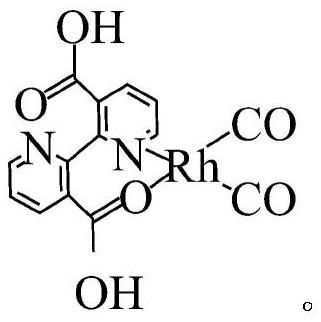
[0029] The pyridyl rhodium catalyst of this embodiment consists of pyridyl-containing compound 2,2'-bipyridine-3,3'-dicarboxylic acid and Rh 2 (CO) 4 Cl 2 Coordination formation, the structural formula of pyridyl rhodium catalyst is as follows:
[0030]
[0031] The preparation method of above-mentioned pyridyl rhodium catalyst comprises the following steps:
[0032] (1) Weigh Rh 2 (CO) 4 Cl 2 Soluble in CH 3 Solution A is obtained in OH solution, and Rh in solution A 2 (CO) 4 Cl 2 The molar concentration is 0.006mol / L;
[0033] (2) According to the molar ratio Rh 2 (CO) 4 Cl 2 : 2,2'-bipyridine-3,3'-dicarboxylic acid=1:2 Weigh 2,2'-bipyridine-3,3'-dicarboxylic acid, dissolve in CH 3 Obtain solution B in OH, the molar concentration of 2,2'-bipyridine-3,3'-dicarboxylic acid in solution B is 0.006mol / L;
[0034] (3) According to the molar ratio Rh 2 (CO) 4 x 2 : NaBPh 4 =1:2 weigh NaBPh 4 , soluble in CH 3 OH to get solution C, in solution C NaBPh 4 The mol...
example 2
[0046] The pyridyl rhodium catalyst of the present embodiment is composed of pyridyl-containing compound 3-methyl-2-pyridinecarboxylic acid and Rh 2 (CO) 4 Cl 2 Coordination formation, the structural formula of pyridyl rhodium catalyst is as follows:
[0047]
[0048] The preparation method of above-mentioned pyridyl rhodium catalyst comprises the following steps:
[0049] (1) Weigh Rh 2 (CO) 4 Cl 2 Soluble in CH 3 Solution A is obtained in OH solution, and Rh in solution A 2 (CO) 4 Cl 2 The molar concentration is 0.004mol / L;
[0050] (2) According to the molar ratio Rh 2 (CO) 4 Cl 2 : 3-methyl-2-pyridinecarboxylic acid=1:2 Weigh 3-methyl-2-pyridinecarboxylic acid, dissolve in CH 3 Solution B is obtained in OH, and the molar concentration of 3-methyl-2-pyridinecarboxylic acid in solution B is 0.004mol / L;
[0051] (3) According to the molar ratio Rh 2 (CO) 4 Cl 2 : NaBPh 4 =1:2 weigh NaBPh 4 , soluble in CH 3 OH to get solution C, in solution C NaBPh 4 T...
Embodiment 3
[0057] The pyridyl rhodium catalyst of the present embodiment is composed of pyridyl-containing compound methyl picolinate and Rh 2 (CO) 4 I 2 Coordination formation, the structural formula of pyridyl rhodium catalyst is as follows:
[0058]
[0059] The preparation method of above-mentioned pyridyl rhodium catalyst comprises the following steps:
[0060] (1) Weigh Rh 2 (CO) 4 I 2 Soluble in CH 3 Solution A is obtained in OH solution, and Rh in solution A 2 (CO) 4 I 2 The molar concentration is 0.008mol / L;
[0061] (2) According to the molar ratio Rh 2 (CO) 4 I 2 : 2,2'-bipyridine-3,3'-dicarboxylic acid=1:2 Weigh 2,2'-bipyridine-3,3'-dicarboxylic acid, dissolve in CH 3 Obtain solution B in OH, the molar concentration of 2,2'-bipyridine-3,3'-dicarboxylic acid in solution B is 0.008mol / L;
[0062] (3) According to the molar ratio Rh 2 (CO) 4 I 2 : NaBPh 4 =1:2 weigh NaBPh 4 , soluble in CH 3 OH to get solution C, in solution C NaBPh 4 The molar concentrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com