Methods for treating severe atopic dermatitis by administering an il-4r inhibitor
A technology of atopic dermatitis and inhibitor, applied in the field of treatment of atopic dermatitis, can solve problems such as unsatisfied
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0100] In embodiment 1, the invention includes a method of treating severe atopic dermatitis (AD) comprising: (a) selecting a patient with severe AD, wherein the patient is resistant, inadequately responsive, or intolerant to systemic immunosuppressant therapy and / or when such treatment is not advisable; (b) administering one or more doses of an interleukin 4 receptor (IL-4R) inhibitor to the patient in need.
[0101] In embodiment 2, the invention includes the method of embodiment 1, wherein the treatment is not advisable due to safety and health risks to the patient and suboptimal efficacy.
[0102] In embodiment 3, the invention includes the method of embodiment 1 or 2, wherein the systemic immunosuppressant therapy is selected from the group consisting of cyclosporin A, methotrexate, mycophenolate mofetil, azathioprine, systemic corticosteroids, and interferon- gamma.
[0103] In embodiment 4, the invention includes the method of embodiment 3, wherein the systemic immunos...
Embodiment approach 13
[0112] In embodiment 13, the invention includes a method of treating severe AD comprising: (a) selecting a patient with severe AD, wherein the patient has previously been treated with a drug selected from the group consisting of cyclosporin A, an IgE inhibitor, a TNFα inhibitor, a CD11a inhibitor , CD20 inhibitors, antibiotics, IL-4R inhibitors, dupilumab, systemic immunosuppressants, topical corticosteroids, oral corticosteroids, calcineurin inhibitors, and phototherapy; and (b) as needed The patient is administered one or more doses of an IL-4R inhibitor.
[0113] In embodiment 14, the invention includes the method of embodiment 13, wherein the patient is resistant, underresponsive, or intolerant to the therapeutic agent.
[0114] In embodiment 15, the invention includes the method of embodiment 13 or 14, wherein the treatment is not advisable due to safety and health risks to the patient and suboptimal efficacy.
[0115] In embodiment 16, the invention includes the method ...
Embodiment 1
[0150] Example 1: Anti-IL-4R Antibodies in Adult Patients with Severe Atopic Dermatitis (AD) Insufficiently Controlled with or Intolerant to Cyclosporine A When Treatment Here is Medically Inadvisable clinical trials in
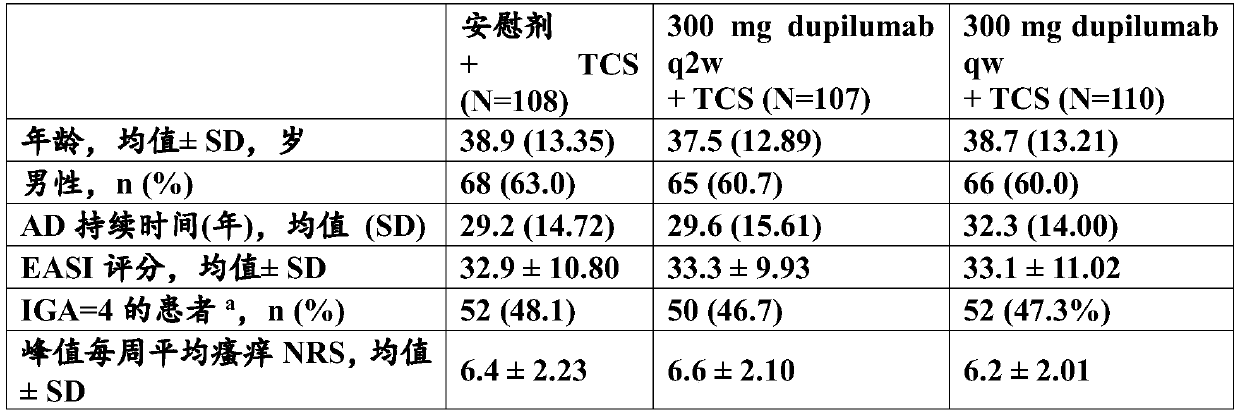
[0151] This is a 32-week double-blind, randomized, placebo-controlled, parallel-group study to confirm the efficacy, safety and tolerability of dupilumab in adults with severe AD. In adults, cyclosporine A (CSA) has not demonstrated sufficient efficacy, has unacceptable side effects, or for whom initial CSA is not medically advisable.
[0152] Dupilumab is a fully human anti-IL-4R antibody, comprising a heavy chain comprising the amino acid sequence of SEQ ID NO:9 and a light chain comprising the amino acid sequence of SEQ ID NO:10; the HCVR / LCVR amino acid sequence pair comprising SEQ ID NO:1 / 2 and heavy and light chain CDR sequences comprising SEQ ID NOs: 3-8.
[0153] The study included a 2-week screening period, a 2-week moderate-power TCS standardizati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com