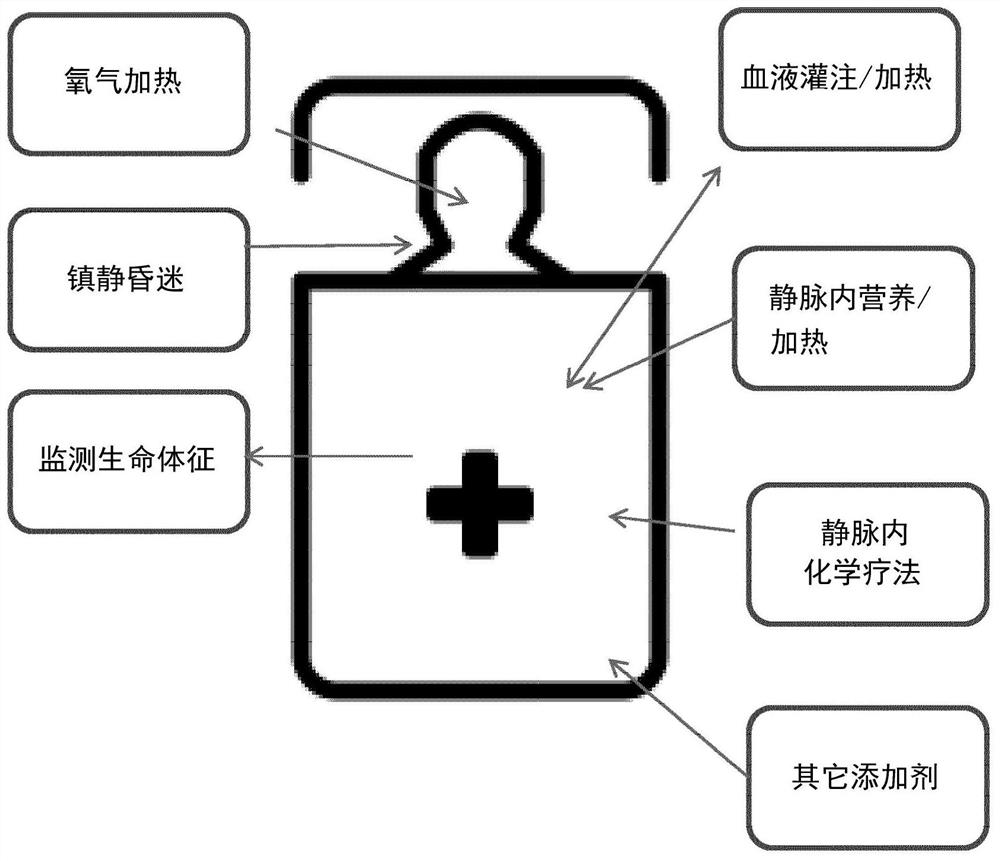
Whole body hyperthermia system
A whole body, body technology, applied in the field of anti-tumor compounds, can solve the problems of lack of safety, small discomfort and high efficiency, and achieve the effect of small damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0213] Example 1. In vitro model for optimization of the first heating device.
[0214] A fibroblast cell line (normal human dermal fibroblasts (NHDF)) and a tumor cell line were incubated in 5% CO 2 Culture in DMEM with fetal bovine serum in an incubator. Suitable cell lines are eg HeLa cells, HT-1080 (connective tissue cell line with activated N-ras oncogene), A375 (malignant cutaneous melanoma cell line) and A549 (adenocarcinoma human alveolar basal epithelial cells).
[0215] Cells were placed in an incubator at 37°C and then the temperature was raised to 41.5°C over a period of time ranging from 30 minutes to 4 hours. The volume of medium on the cells is kept to a minimum to avoid delays in heating.
[0216] Determine cell viability and measure apoptosis and necrosis of cells.
[0217] This experiment allowed to determine the effect of a slow or sharp increase in temperature achieved by the first device when applied on the body.
example 2
[0218] Example 2. In vitro model for determining efficiency and specificity.
[0219] The above cells were cultured at different temperatures (37, 39, 40, 40.5, 41, 41.5 and 42° C.) for different time periods (4, 6, 8, 12, 16, 20, 24, 36 hours).
[0220] Determine cell viability and measure apoptosis and necrosis of cells.
[0221] This experiment allowed to determine the efficiency (percentage of tumor cells killed) and specificity (percentage of tumor cells killed versus normal cells killed) of the method.
[0222] The system described above can further be used to simulate conditions (hypoxia, pH) in tumor tissue and determine the effect on cells that have been treated or irradiated with cancer drugs.
example 3
[0223] Example 3. In vivo model for long-term hyperthermia.
[0224]Place tumor-bearing mice under anesthesia on a heating plate in a heating cabinet. For small laboratory animals, heat transfer is sufficient to provide whole body hyperthermia without the use of the first and second devices as described in the specification.
[0225] Mice were sacrificed and tumor tissues were examined for cell death.
[0226] The animal model allows comparing the cell death obtained in Example 2 and the mouse model using the same time period and the same temperature.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com