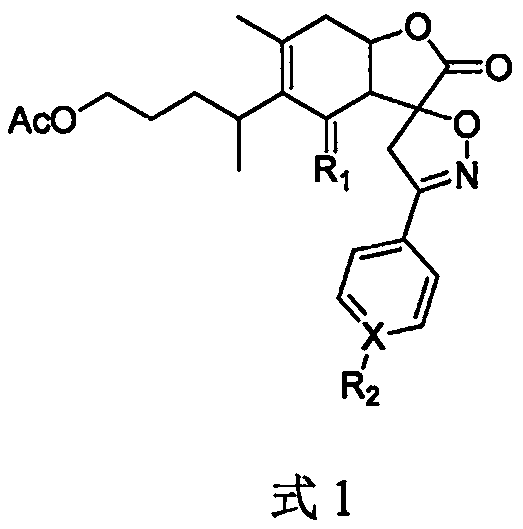
1-o-acetyl inulalide spiroaryl isoxazoline derivatives and their medicinal uses
A technology of inula inula, isoxazoline, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1: the preparation method of compound 1a-1j
[0016] A certain volume of acetonitrile and water (V / V=1:1) dispersed the aromatic aldehyde in the reaction flask, and added hydroxylamine hydrochloride (1.1 equivalents) into the reaction flask at room temperature. The reaction solution was stirred at room temperature and monitored by TLC until the reaction was complete. The reaction solution was concentrated under reduced pressure to obtain a crude intermediate without purification, which was directly carried out in subsequent reactions. The intermediate obtained in the above reaction was dissolved in DMF at 40°C and stirred, and 1 equivalent of N-chlorosuccinimide (NCS) was added in batches, and the reaction solution was stirred at 40°C under heat preservation conditions, and TLC was monitored until the reaction was complete. 20 times the amount of EtOAc was added to the solution to dilute the reaction solution, the reaction solution was washed with water (5×2...
Embodiment 2
[0017] Embodiment 2: the preparation method of compound 2a-2j
[0018] Use 0.75 mL of CH 2 Cl 2 Disperse intermediate 1a-1j (0.16mmol) in a reaction flask, add 0.13mmol of triethylamine (Et3N) with stirring at room temperature, use 0.75mL of CH 2 Cl 2 Dissolve 1-O-acetyl inulalide (0.1 mmol), add the solution into the reaction flask, and stir for reaction. After 16 hours of reaction, TLC monitors that the reaction is complete, and directly concentrates the reaction solution under reduced pressure to obtain a crude product. 2a-2j were obtained by silica gel column chromatography (300-400 mesh, PE:EA=6:1-3:1, V / V).
[0019] 2.1 Synthesis of Compound 2a
[0020] 2a, colorless oil, yield 44.7%. 1 H NMR (400MHz, CDCl 3 ): δ7.69(d, J=7.4Hz, 1.4Hz, 2H), 7.40-7.47(m, 3H), 5.17(m, 1H), 4.13(s, 1H), 4.01(t, J=6.3Hz , 2H), 3.63(s, 2H), 2.93(dd, J=5.9Hz, 2.0Hz, 1H), 2.72-2.77(m, 2H), 2.48-2.53(m, 1H), 2.01(s, 3H) , 1.78(s, 3H), 1.42-1.57(m, 2H), 1.19-1.40(m, 2H), 1.13(d, J=7.0Hz, ...
Embodiment 3
[0039] Embodiment 3: the preparation method of compound 3a-3j
[0040] Use 3 mL of anhydrous dichloromethane (freshly prepared) to dissolve DMAP (1.1 equivalents) and acetic anhydride (1.5 equivalents) in a reaction flask, and 3 mL of dichloromethane to dissolve 1-O-acetyl inulalide (1 equivalents ), the solution was added to the reaction bottle, and transferred to room temperature for reaction. After 20 minutes, TLC monitored the reaction to be complete, and the reaction was stopped. 10 mL of ice water was added to the reaction bottle and stirred for 20 minutes. The reaction liquid was extracted with dichloromethane (15 mL×3), anhydrous MgSO 4 Dry, filter, and concentrate under reduced pressure to obtain crude 1,6-O,O-acetyl inulalide. The crude product was purified by silica gel column chromatography to obtain a pure product for subsequent reactions. Use 0.3 mL of CH 2 Cl 2 Disperse intermediate 1a-1j (0.16mmol) in the reaction bottle, stir at room temperature and add 0.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com