Method for electrochemically preparing tridecane from azelaic acid
A technology of tridecane and azelaic acid, applied in the field of electrochemical synthesis, can solve the problem of no one trying electrochemical synthesis method, etc., and achieve the effects of green reaction process, short reaction period and low reaction cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
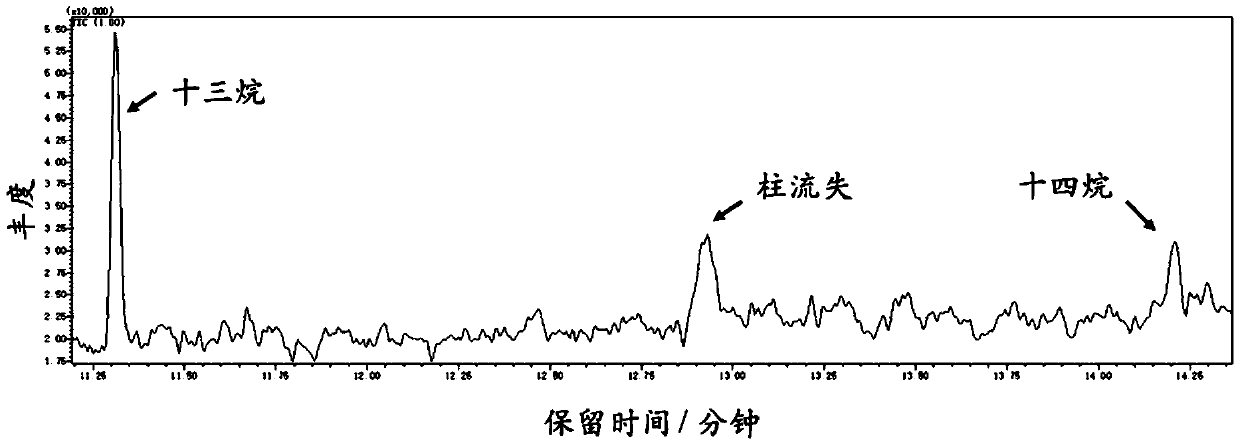
[0025] Drugs: Deionized water (18.2MΩ, Merck Milli-QAdvantageA10 ultrapure water system), azelaic acid, potassium hydroxide and potassium sulfate used were bought back and used directly without further purification.
[0026] Electrolytic cell: The electrolytic cell used in this reaction is 50mm 3 The screw port can be sealed without diaphragm Pikes glass electrolytic cell. The electrolysis system is a three-electrode system, in which a Pt sheet (1cm×1cm) is used as the working electrode, a Pt mesh (60 mesh, 1cm×1cm) is used as the counter electrode, and a Hg / HgO (1M KOH) electrode is used as the reference electrode.
[0027] Add 2.35 g of azelaic acid and 6.25 ml of 2 mol / L KOH aqueous solution (as a supporting electrolyte) into the beaker and mix in turn, then add deionized water to make the volume to 25 ml. Put the magnet into the beaker, turn on 600rpm and stir for 10min, then add it to the above electrolytic cell. Place the electrolytic cell in a water bath at about 30°C...
Embodiment 2-25
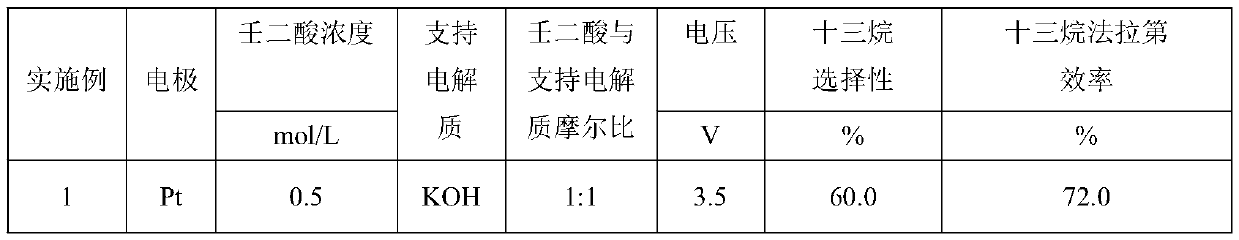
[0030] The influence of the concentration of azelaic acid, solvent type, type of supporting electrolyte, concentration and applied voltage on the selectivity of tridecane is shown in Table 1. The electrolytic cell used in Example 23 is a double-chamber electrolytic cell with a diaphragm, and the diaphragm used is an AMI-7001 anion exchange membrane. Others are the same as embodiment 1.
Embodiment 1-25
[0032]
[0033]
[0034] It can be seen from the data in Table 1 that when the solvent is deionized water and other conditions are certain, the higher the concentration of azelaic acid, the higher the Faradaic efficiency and hydrocarbon selectivity, but the performance is not obvious, and azelaic acid is generally selected The acid concentration is 0.5mol / L. Under certain other conditions, the supporting electrolyte is alkaline, and the Faraday efficiency and tridecane selectivity are high. The stronger the alkalinity, the higher the Faraday efficiency and tridecane selectivity. Considering other factors, KOH is generally selected, and Its molar ratio to azelaic acid is 1:1. It is known from Examples 1 and 22 that under certain other conditions, when the reaction is carried out in a single-chamber electrolytic cell without a diaphragm, its Faraday efficiency and hydrocarbon selectivity are lower than those carried out in a double-chamber electrolytic cell with a diaphrag...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com