Sulfur-sulfur bond containing dipolymacrocyclic nickel (II) complex and in-situ synthesis method thereof
A technology of in-situ synthesis and complexes, which is used in pharmaceutical formulations, medical preparations with inactive ingredients, drug combinations, etc., to achieve the effect of wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0016] A method for in-situ synthesis of dimeric macrocyclic nickel (II) complexes containing sulfur-sulfur bonds, comprising the steps of:
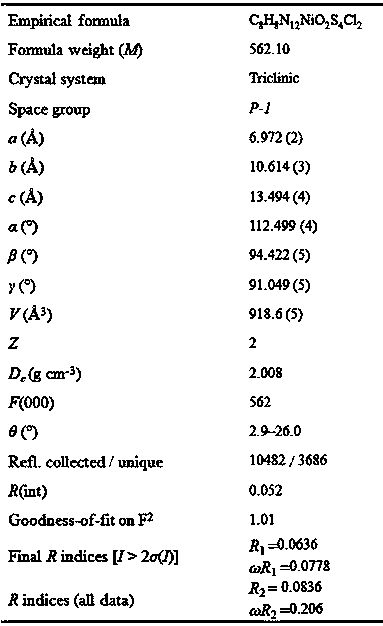
[0017] (1) Weigh 0.0400g 3,3’-bis(5-mercapto-1,2,4-triazole) (H 4 L) and 0.0475g nickel dichloride hexahydrate NiCl 2 ·6H 2 O, then added to 4mL methanol and 6mL 1,4-dioxane, and the mixture was stirred and reacted at room temperature for 6h;
[0018] (2) Filter the above mixed solution, put the filtrate in a beaker, and let it volatilize naturally at room temperature to obtain single crystal grade purple flaky crystals.
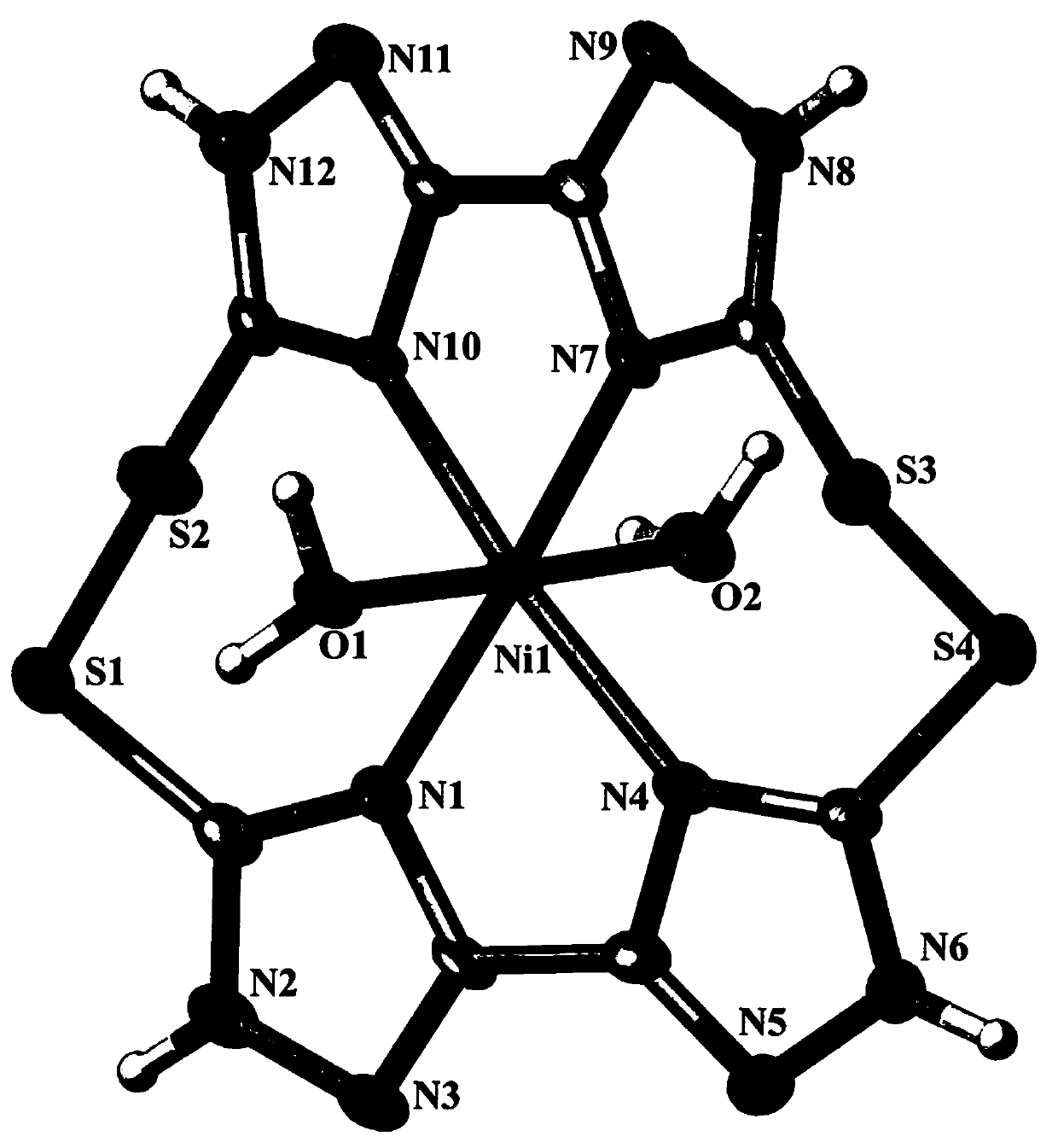
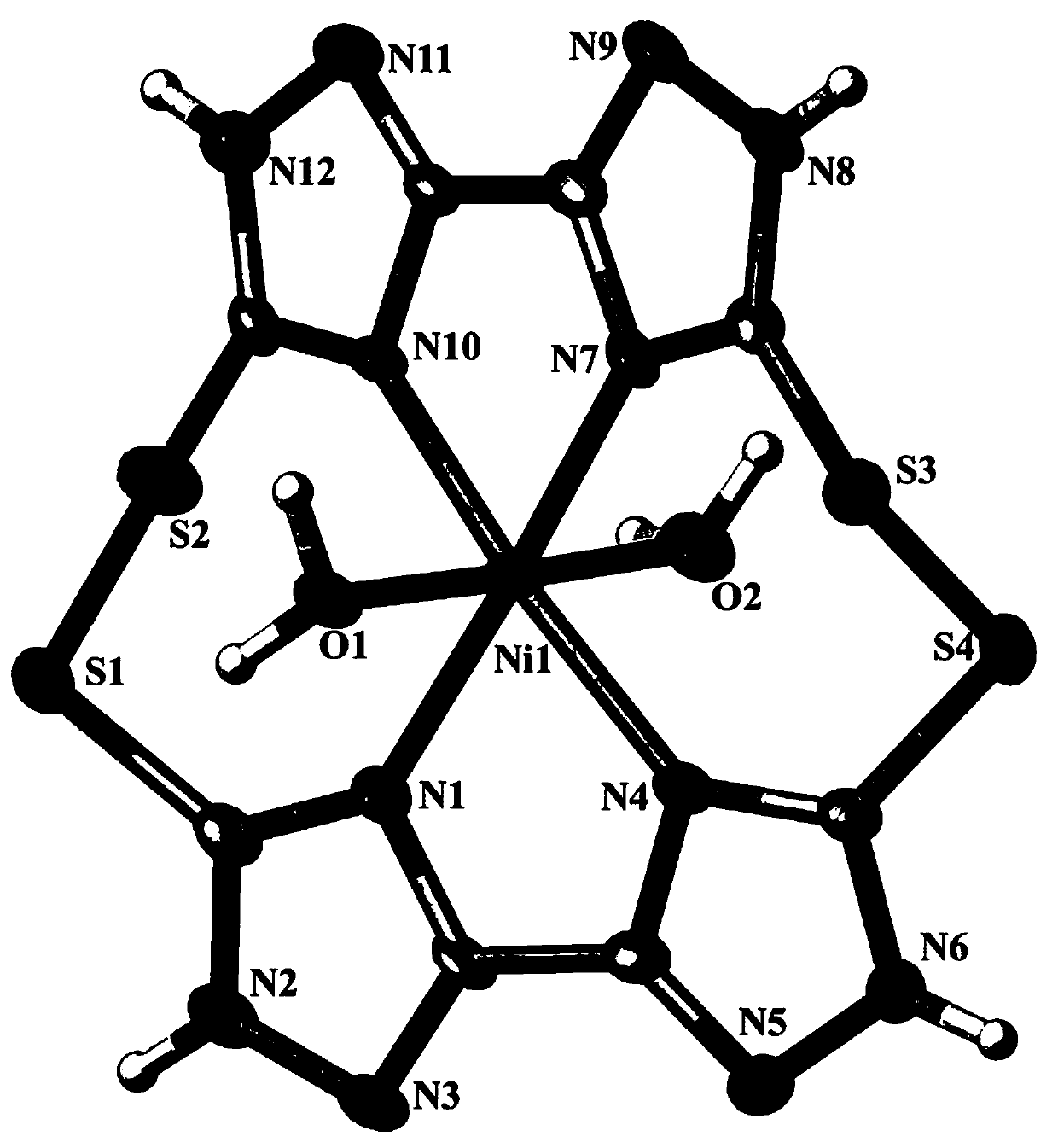
[0019] see figure 1 , Example Schematic diagram of the molecular structure of dimeric macrocyclic nickel (II) complexes containing sulfur-sulfur bonds. For the convenience of observation, the two external chloride ions are deleted. It can be clearly seen from the figure that the inner boundary of the complex is composed of a Ni 2+ , a macrocyclic ligand (H 4 L 1 ) and two H 2 O molecular composition, Ni 2+ io...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com