Synthetic method of 3-benzylidene isoindoline-1-ketone derivative
A technology of benzylidene isoindoline and a synthesis method, which is applied in the field of pharmaceutical chemical intermediates and related chemical synthesis, can solve the problems of poor substrate applicability, harsh reaction conditions, restricted application and the like, and achieves cost reduction and good universality. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
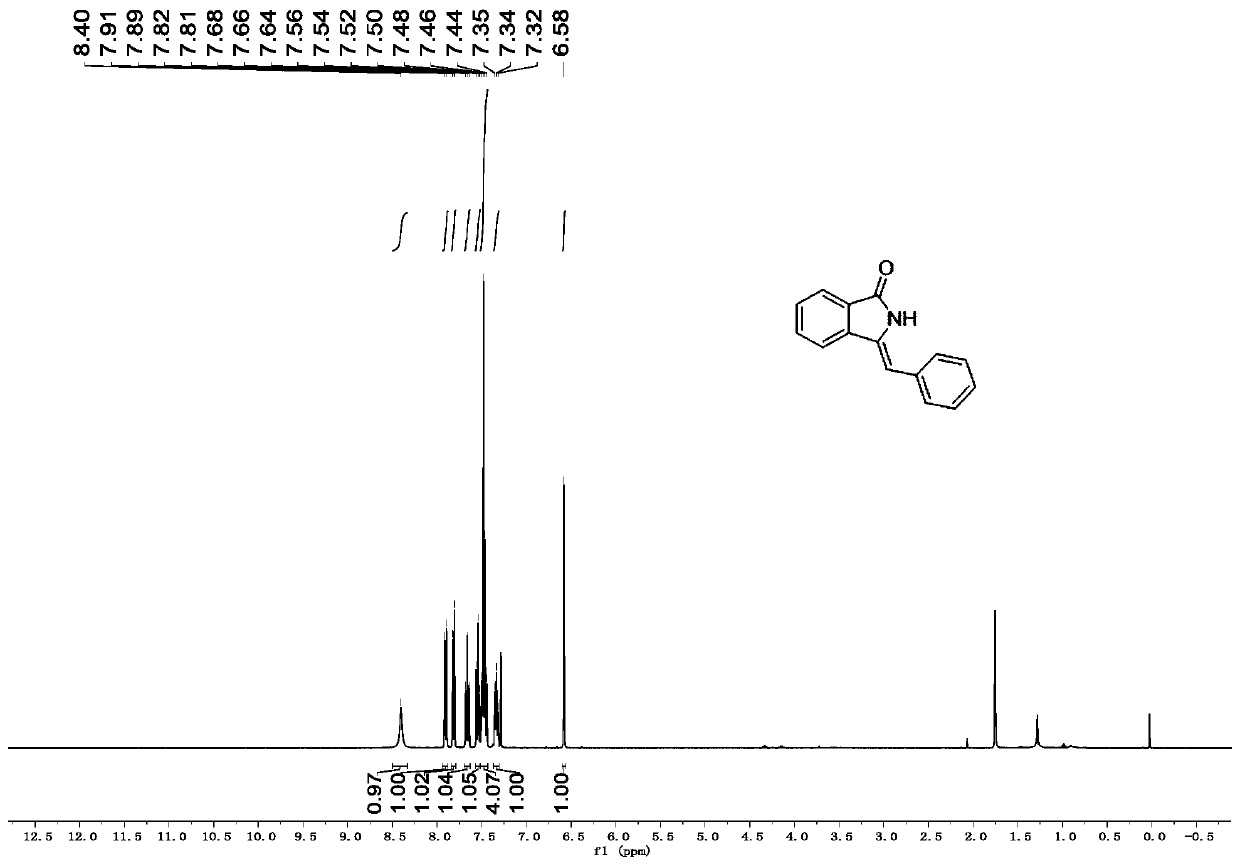
[0039] Synthesis of (3-(benzylidene)isoindolin-1-one)
[0040] In a 25mL reactor, add o-bromoaniline (0.034g, 0.2mmol), cuprous iodide (0.004g, 0.02mmol) and potassium tert-butoxide (0.045g, 0.4mmol), replace nitrogen with vacuum three times and then add benzene Acetylene (0.031g, 0.3mmol) and water (0.011g, 0.6mmol) were added to anhydrous 1,4-dioxane 1.5mL and stirred at 100°C for 12h. Column chromatography (silica gel, 200-300 mesh; developing solvent, petroleum ether: ethyl acetate = 5:1) obtained 0.028 g of 3-(benzylidene)isoindolin-1-one with a yield of 64%.
[0041]
[0042] white solid, 1 H NMR (500MHz, CDCl 3 )δ8.40(s,1H),7.90(d,J=7.6,1H),7.82(d,J=7.7Hz,1H); 7.66(t,J=7.6Hz,1H),7.54(t,J =7.5Hz,1H),7.51-7.42(m,4H),7.37-7.31(m,1H),6.58(s,1H). 13 CNMR (125MHz, CDCl 3)δ169.0, 138.2, 135.0, 133.1, 132.3, 129.2, 129.1, 128.7, 128.5, 127.7, 123.6, 119.8, 105.9.
Embodiment 2
[0044] Synthesis of (3-(3-bromobenzylidene)isoindolin-1-one)
[0045] In a 25mL reactor, add o-bromoaniline (0.034g, 0.2mmol), cuprous iodide (0.004g, 0.02mmol) and potassium tert-butoxide (0.045g, 0.4mmol), replace nitrogen with vacuum three times and then add 3 -Bromophenylacetylene (0.054g, 0.3mmol) and water (0.015g, 0.8mmol), add anhydrous 1,4-dioxane 1.5mL, and stir at 110°C for 16h. Column chromatography (silica gel, 200-300 mesh; developing solvent, petroleum ether: ethyl acetate = 5:1) obtained 0.042 g of 3-(3-bromobenzylidene) isoindolin-1-one, the yield 70%.
[0046]
[0047] Pale yellow solid, 1 H NMR (500MHz, DMSO) δ10.90(s, 1H), 8.04(d, J=7.8Hz, 1H), 7.86-7.67(m, 3H); 7.67-7.54(m, 2H), 7.46(d, J=8.0Hz, 1H), 7.36(d, J=15.7Hz, 1H), 6.74(s, 1H). 13 CNMR (125MHz, DMSO) δ174.4, 143.7, 142.3, 138.8, 137.6, 136.4, 135.9, 135.0, 134.7, 133.5, 133.2, 127.9, 127.4, 125.7, 109.3.
Embodiment 3
[0049] Synthesis of (3-(4-Propylbenzylidene)isoindolin-1-one)
[0050] The operation was the same as in Example 1, and 0.041 g of 3-(4-propylbenzylidene)isoindolin-1-one was obtained by reacting o-bromobenzonitrile and 4-propylphenylacetylene, with a yield of 77%.
[0051]
[0052] white solid, 1 H NMR (500MHz, CDCl 3 )δ8.33(s,1H),7.90(d,J=7.6Hz,1H),7.80(d,J=7.8Hz,3H),7.69-7.61(m,1H),7.53(t,J=7.4 Hz,1H),7.40(d,J=8.1Hz,1H),7.27(d,J=8.2Hz,2H),6.56(s,1H),2.68-2.60(t,J=8.0Hz,2H), 1.69(m,2H),0.99(t,J=7.3Hz,3H). 13 C NMR (125MHz, CDCl 3 )δ168.9, 142.6, 138.3, 132.5, 132.4, 129.4, 129.0, 128.7, 128.4, 123.6, 119.7, 106.2, 37.8, 24.4, 13.8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com