Method for preparing Co9S8 and nitrogen doped carbon composite array electrode
A composite array, nitrogen-doped carbon technology, applied in the direction of electrodes, chemical instruments and methods, electrolysis process, etc., can solve the problems of lack of inherent active sites, small specific surface area of nanoparticles, poor stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
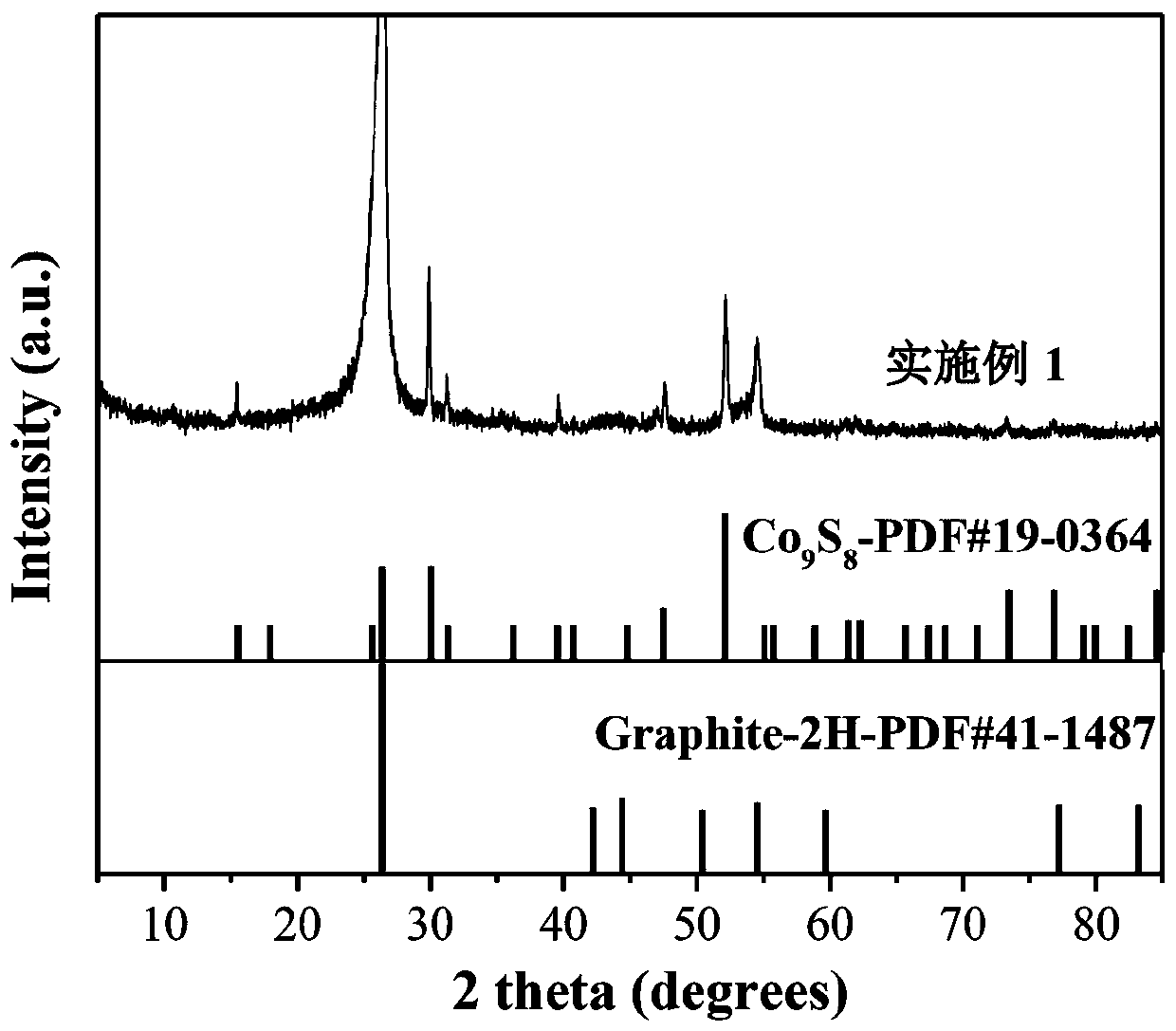
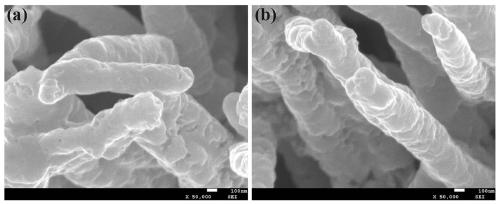
[0020] At room temperature, 0.15 M CoCl 2 ∙ 6H 2 O, urea with a mass fraction of 6.25% was dissolved in 40 mL of deionized water. The carbon paper was soaked in the solution and reacted in a water bath at 90 °C for 2 h, cooled naturally to room temperature, the carbon paper was taken out, rinsed with deionized water three times, and dried for later use. Soak the carbon paper prepared above in 50 mL of Tris base with a concentration of 0.01 M and a pH of 8.5, add 40 mg of dopamine, stir at room temperature for 24 h, rinse the sample three times with deionized water and dry it. The carbon paper was put into a tube furnace, reacted at 350 °C for 2 h under the airflow of thiourea (0.2 g), then continued to heat up to 700 °C for 2 h, cooled naturally to room temperature and took it out to obtain CFP / Co 9 S 8 @C in situ electrodes.
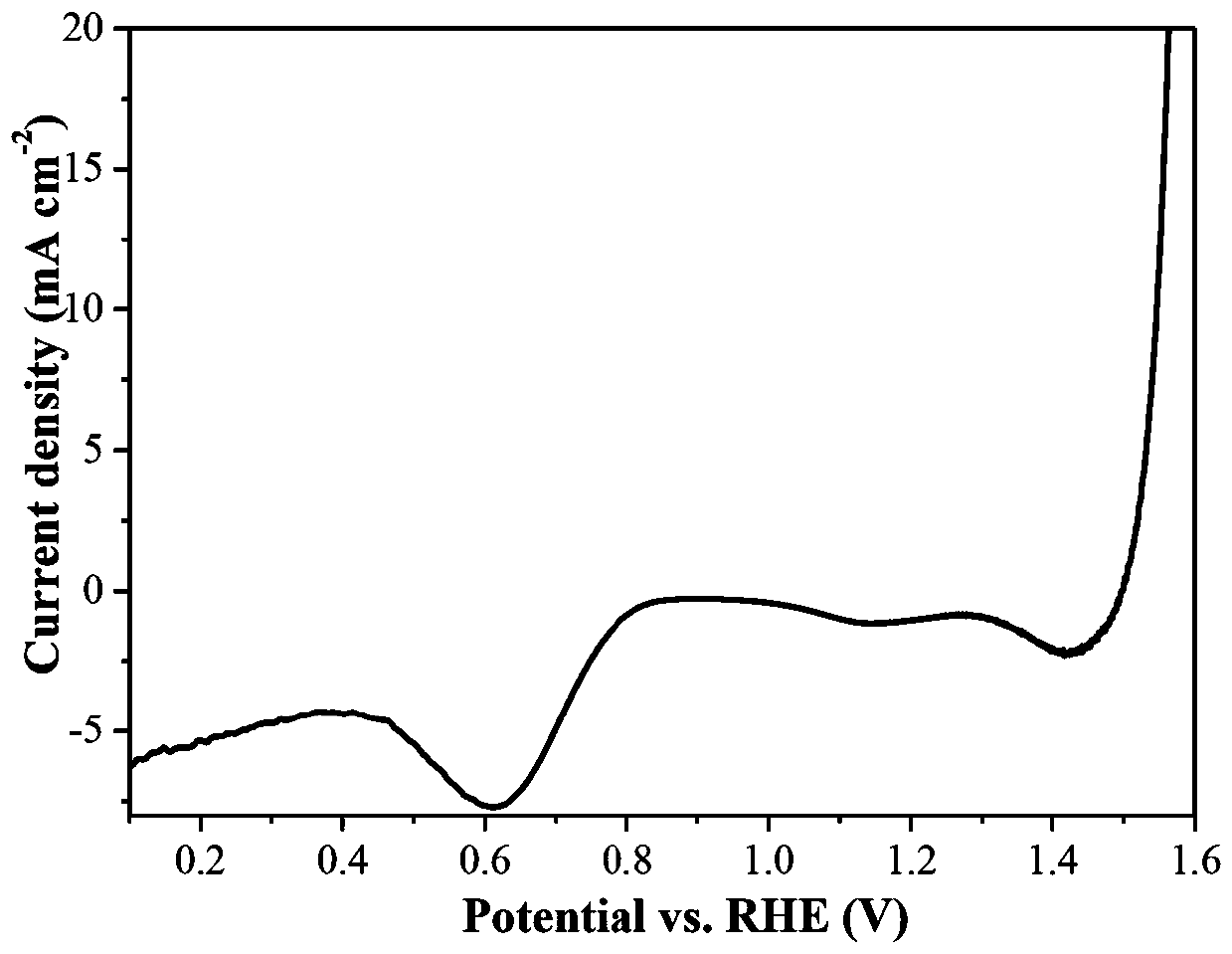
[0021] figure 1 The OER and ORR linear voltammetry sweep (LSV) diagrams of the electrode prepared in Example 1. It can be seen from the figure t...
Embodiment 2
[0025] At room temperature, 0.15 M CoCl 2 ∙ 6H 2 O, 6.25% urea was dissolved in 40 mL deionized water. The carbon paper was soaked in the solution and reacted in a water bath at 90 °C for 2 h, cooled naturally to room temperature, the carbon paper was taken out, rinsed with deionized water three times, and dried for later use. Soak the carbon paper prepared above in 50 mL of Tris base with a concentration of 0.01 M and a pH of 8.5, add 30 mg of dopamine, stir at room temperature for 24 h, rinse the sample three times with deionized water, and then dry it. The carbon paper was put into a tube furnace, reacted at 350 °C for 2 h under the airflow of thiourea (0.2 g), then continued to heat up to 700 °C for 2 h, cooled to room temperature naturally, and took out to obtain CFP / Co 9 S 8 @C in situ electrodes.
[0026] Figure 4The OER and ORR linear voltammetry sweep (LSV) diagrams of the electrode prepared in Example 2. It can be seen from the figure that when the current de...
Embodiment 3
[0028] At room temperature, 0.15 M CoCl 2 ∙ 6H 2 O, 6.25% urea was dissolved in 40 mL deionized water. Soak the carbon paper in the solution and react it in a 90°C water bath for 2 hours, cool it down to room temperature naturally, take out the carbon paper, rinse it with deionized water three times, and dry it for later use. Soak the carbon paper prepared above in 50 mL of Tris base with a concentration of 0.01 M and a pH of 8.5, add 50 mg of dopamine, stir at room temperature for 24 h, rinse the sample three times with deionized water, and then dry it. The carbon paper was put into a tube furnace, reacted at 350 °C for 2 h under the air flow of thiourea (0.2 g), then continued to heat up to 700 °C for 2 h, cooled naturally to room temperature and took it out to obtain CFP / Co 9 S 8 @C in situ electrodes.
[0029] Figure 5 The OER and ORR linear voltammetry (LSV) diagrams of the electrode prepared in Example 3. It can be seen from the figure that when the current densi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Limiting current density | aaaaa | aaaaa |
| Limiting current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com