Sarcoma fusion gene detection kit and system
A detection kit and gene fusion technology, applied in the field of medical detection, can solve the problems of inconsistency in diagnosis, difficulty in diagnosis, affecting treatment plan formulation and prognosis judgment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] A sarcoma fusion gene detection kit, comprising the following primer sets:
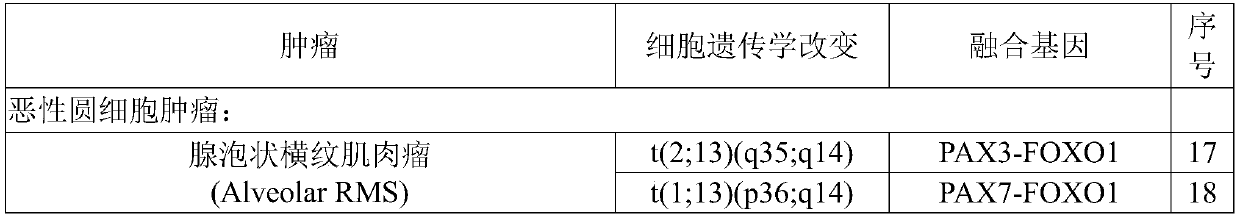
[0056] Table 3. Fusion gene pairs and primer sets
[0057]
[0058]
[0059]
[0060]
[0061]
[0062]
[0063]
[0064] The above primer pair is one equivalent primer pair corresponding to each fusion site, such as COL1A1-PDGFB.C15P2.COSF1293 and COL1A1-PDGFB.C16P2.COSF595 and COL1A1-PDGFB.C16P2.COSF596 use the same primer pair, you need to follow SEQ 3 times of the primer pair of ID No.1-2 was added to the primer pair of SEQID No.9-10.
[0065] The kit of the present embodiment also includes the following internal reference gene primer sets:
[0066] Table 4. Internal reference gene primer set
[0067]
Embodiment 2
[0069] A sarcoma fusion gene detection system, comprising the following modules:
[0070] The detection module adopts the kit of Example 1 for high-throughput sequencing;
[0071] The analysis module obtains the above sequencing results, and obtains the fusion gene detection results through bioinformatics analysis;
[0072] The quality control module is verified by the predetermined quality control program. If the verification is passed, the test result will be output. If the verification fails, the sample will be invalid.
[0073] The workflow of the above detection system is as follows:
[0074] 1. Detection.
[0075] 1. RNA extraction
[0076] RNA was extracted from FFPE (formalin-fixed, paraffin-embedded) tissues.
[0077] 1.1 Preparation before experiment
[0078] a. Unless otherwise specified, all centrifugation is carried out at room temperature, and the centrifugation speed is 13200rpm;
[0079] b. FFPE tissue dosage: 4 pieces of 10μm thickness, surface area 2 (1...
Embodiment 3
[0201] Methodological validation of fusion gene detection in sarcoma.
[0202] 1. Sample source.
[0203] 1. 18 samples from the CAP Sarcoma Translocation PT project, for the official (CAP) confirmation of the status of the fusion gene of the sample.
[0204] 2. Clinical collection of Sarcoma FFPE samples: a total of 21 cases, 1 to 3 slides, all samples have been tested by FISH, and the test results are shown as follows:
[0205] Table 10. Information about 21 clinical SARC FFPE samples
[0206]
[0207]
[0208] The criteria for judging the above FISH test results are as follows:
[0209] 1) Analyze 200 cells, if the number of cells with positive signal is greater than 25%, it is judged to be positive for rearrangement;
[0210] 2) If the number of cells with positive signal is less than 10%, it is judged as rearrangement negative;
[0211] 3) Samples with a positive signal between 10% and 25% are re-tested; samples with a positive signal greater than 15% in the sec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com