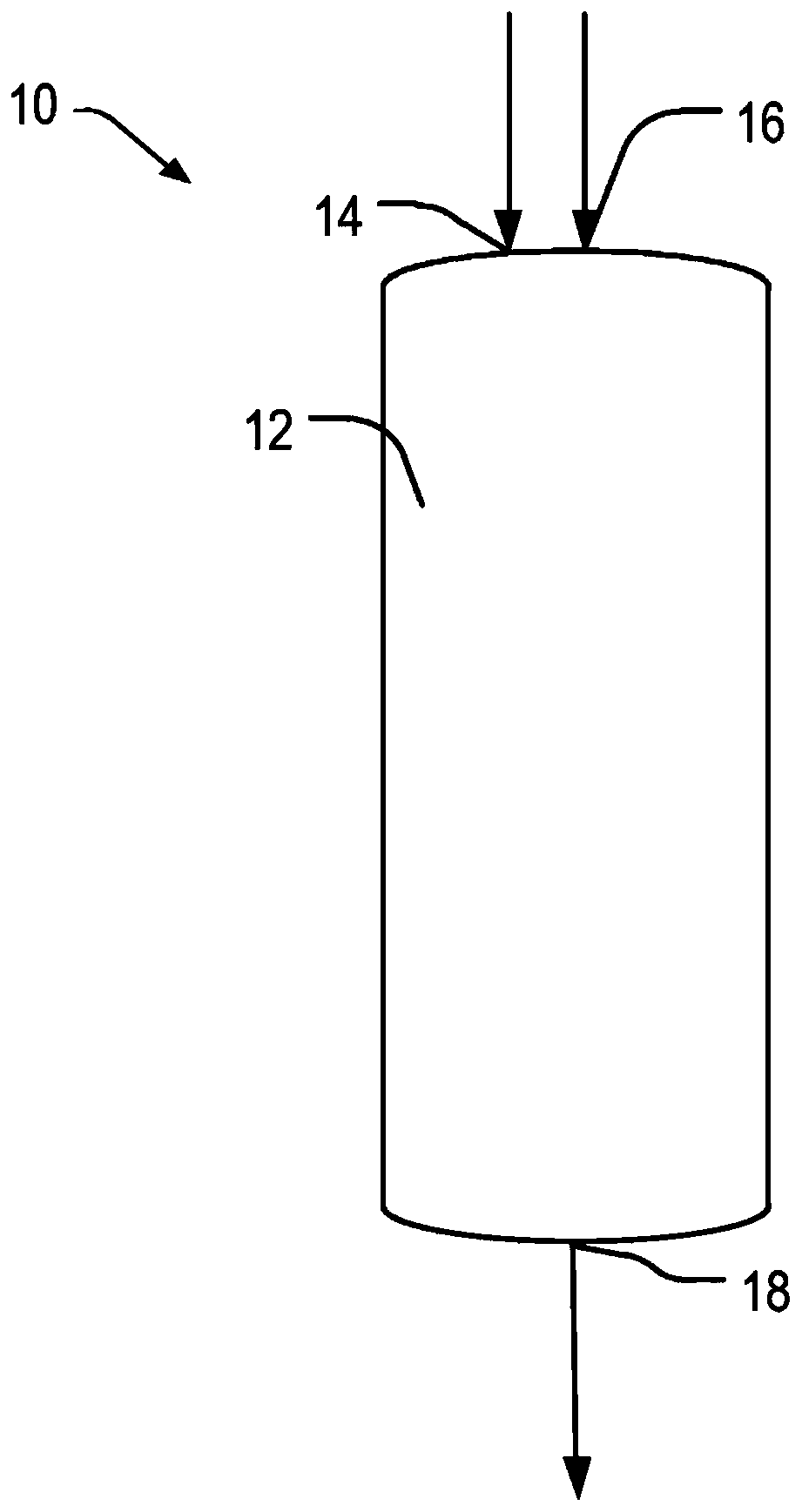
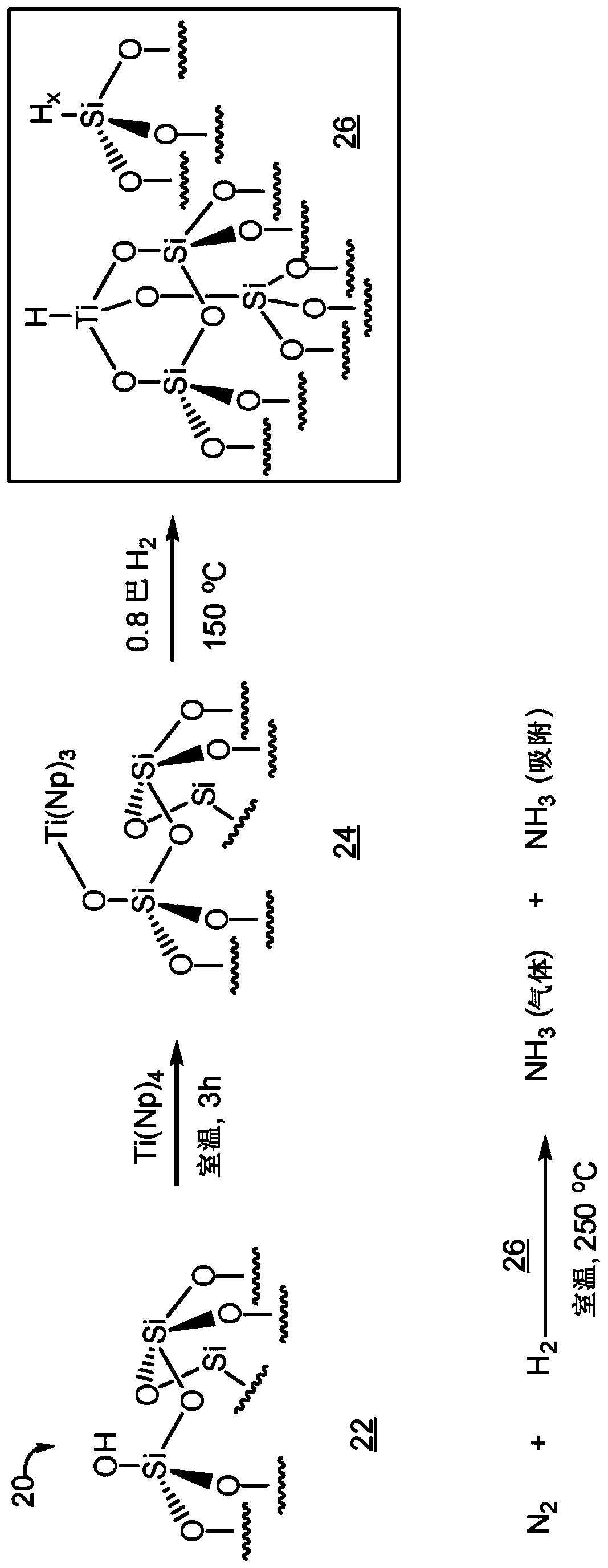
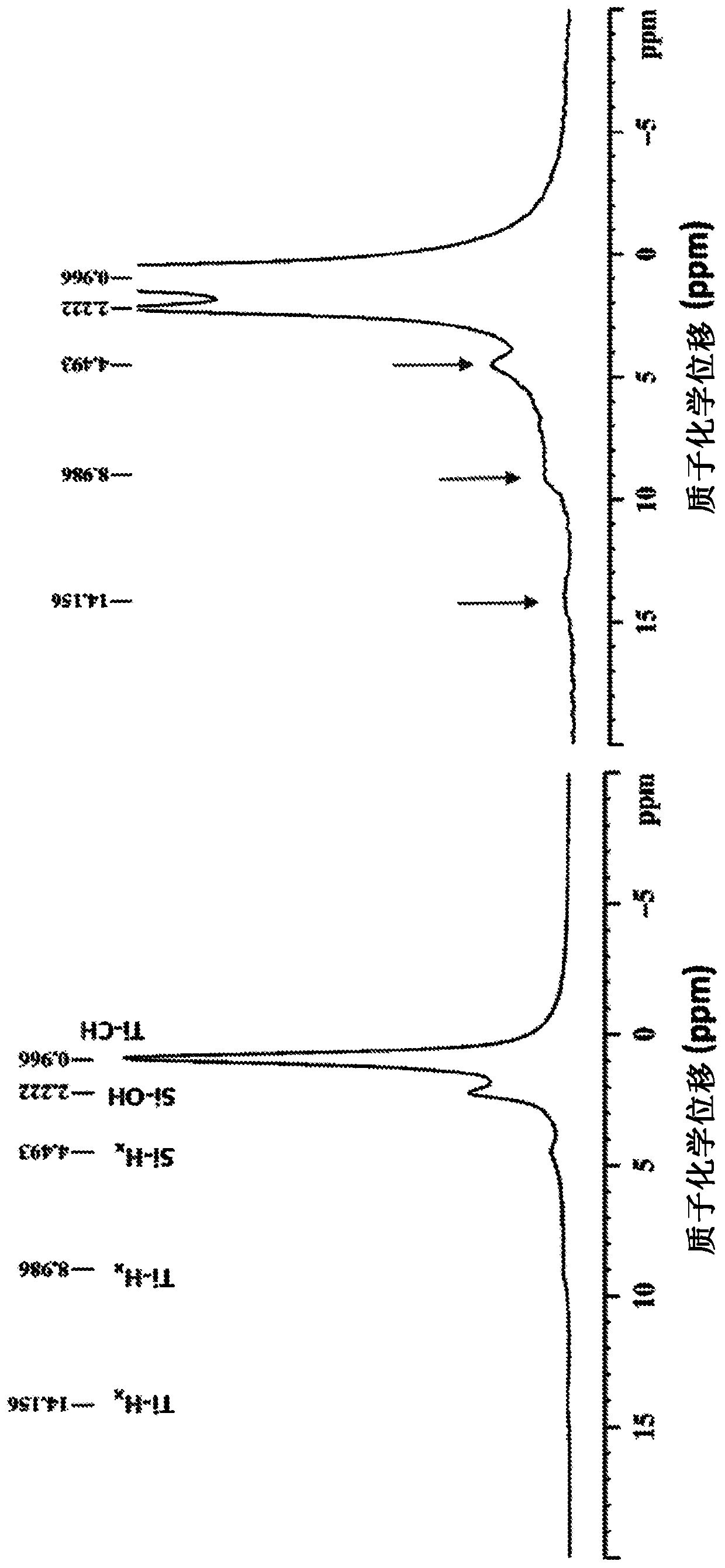
Heterogeneous catalysts/process based on supported/grafted transition metal hydrides for ammonia formation from nitrogen and hydrogen
A transition metal, hydride technology, applied in the field of ammonia preparation, can solve problems such as difficult practical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0086] 1. Preparation of Metal Hydride Materials
[0087]In one embodiment, a method of preparing the metal hydride materials of the invention is described. The first step of the process consists in obtaining a solution containing a hydrocarbon salt comprising a hydrocarbon anion (R - ) and metal cations. hydrocarbon anion (R - ) may be an alkyl or substituted alkyl, including any of the aforementioned alkyl or substituted alkyl. The cations may be lithium ions, sodium ions, potassium ions, magnesium ions, zinc ions, and the like. Non-limiting examples of hydrocarbon salts include alkyllithium, alkylmagnesium halide, dialkylmagnesium, alkylzinc halide, dialkylzinc, etc., which can be purchased from commercial sources or prepared under anhydrous conditions by methods known in the art. Prepared below. In one non-limiting example, the hydrocarbon anion (R - ) is neopentyl lithium prepared by reacting neopentyl chloride and chopped Li metal wires in hexane. In step 2 of the...
Embodiment 1
[0109] (neopentyl lithium (LiCH 2 CMe 3 ) preparation)
[0110]Neopentyllithium was prepared according to Davidson et al. (Organomet. Chem. 1973, 57, 269) by reacting neopentyl chloride (10 g) and chopped Li wire (3 g, 1% Na) in hexane to give 5g to 6g neopentyllithium (70% to 80% yield).
Embodiment 2
[0112] (tetraneopentyl peptide, TiNp 4 or [Ti(CH 2 CMe 3 ) 4 ] preparation)
[0113] According to Clark et al., J.Am.Chem.Soc., 1978, 100, 6774 and McCullough et al., J.Am.Chem.Soc., 1985, 107, 5987, to a solution of neopentyllithium in pentane (50 mL 0.65M solution, 32.5 mmol) Ti(OEt) 4 (1.5 mL, 7.3 mmol) in pentane (20 mL). The reaction mixture was stirred at -78°C for 1.5 hours and then at room temperature for 6 hours. The solvent was then removed to give a brown solid which sublimed over 10 hours (55°C, 10 -3 , to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com