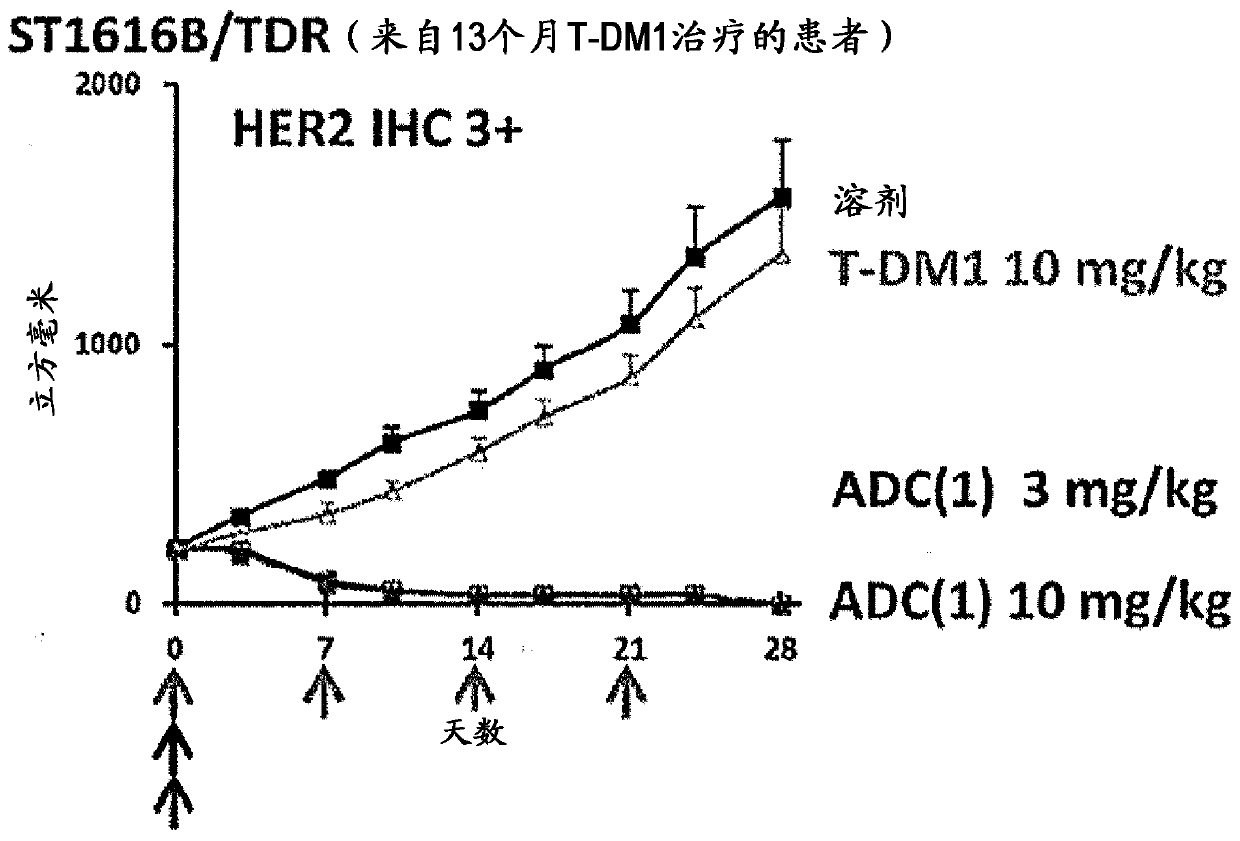
Therapy for drug-resistant cancer by administration of anti-HER2 antibody/drug conjugate
A technology of drug conjugates and conjugates, applied in the direction of antibody medical components, antibodies, drug combinations, etc., can solve the problems of insufficient adaptability of responsiveness and activity intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0424] (1) Antigen preparation
[0425] Examples of antigens for producing anti-HER2 antibodies include HER2 or polypeptides containing partial amino acid sequences of at least 6 consecutive amino acids, or derivatives obtained by adding arbitrary amino acid sequences and carriers to them.
[0426] HER2 can be directly purified and used from human tumor tissue or tumor cells, or can be obtained by synthesizing HER2 in vitro or producing HER2 in host cells through genetic manipulation.
[0427] In genetic manipulation, specifically, after integrating the cDNA of HER2 into an expressible vector, it is synthesized in a solution containing enzymes, substrates and energy substances required for transcription and translation, or other prokaryotes or Eukaryotic host cells are transformed to express HER2, and antigens can be obtained.
[0428] Alternatively, the antigen can also be obtained as a secreted protein by expressing a fusion protein obtained by linking the extracellular reg...
Embodiment
[0671] The present invention will be specifically described by the examples shown below, but the present invention is not limited by them. Also, these examples are not to be construed as limiting in any way.
manufacture example
[0672] [Manufacturing example: Preparation of antibody-drug conjugate]
[0673] According to the production method described in Patent Document 8 (International Publication No. 2015 / 115091), an antibody-drug conjugate represented by the following formula (hereinafter referred to as "antibody-drug conjugate (1)" or "ADC (1) )”).
[0674] [chemical formula 28]
[0675]
[0676] Here, the drug-linker structure is connected to the antibody through a thioether bond, and n is in the range of 7-8.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com