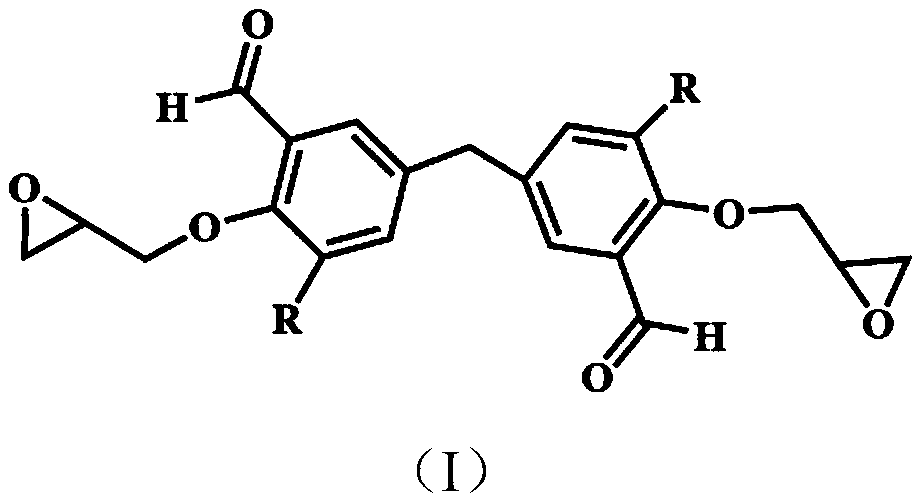
A kind of bio-based flame retardant epoxy resin precursor based on natural phenolic monomer and its preparation method and application
An epoxy resin, bio-based technology, applied in the direction of organic chemistry, can solve the problems that epoxy resin is not a pure bio-based, green, environmentally friendly product, complex preparation method, harsh reaction conditions, etc., to achieve sustainable sources The development and synthesis steps are simple, and the mechanical properties and flame retardant properties are good
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Dissolve 1mol of salicylaldehyde, 0.5mol of paraformaldehyde, and 0.01mol of sulfuric acid in 200mL of anhydrous acetic acid, react at 60°C for 40 hours, remove the solvent by rotary evaporation under reduced pressure, wash with water and dry to obtain dihydrate Popylaldehyde compound, the productive rate is 89%;
[0034] (2) React 1mol disalicylaldehyde compound and 10mol epichlorohydrin in the presence of 0.1mol tetrabutylammonium bromide at 80°C for 12 hours, then remove the solvent by rotary evaporation under reduced pressure, wash with water and dry The epoxidized disalicylaldehyde compound was obtained with a yield of 95%.
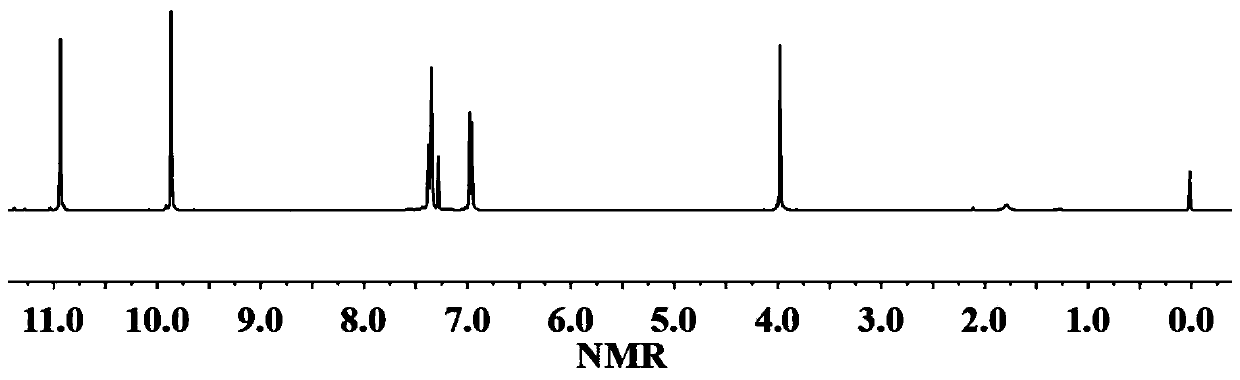
[0035] The proton nuclear magnetic resonance spectrum of the disalicylaldehyde compound that step (1) obtains 1 H-NMR such as figure 1 As shown, each peak on the figure is in one-to-one correspondence with the hydrogen atoms on the structure of the disalicylaldehyde compound.
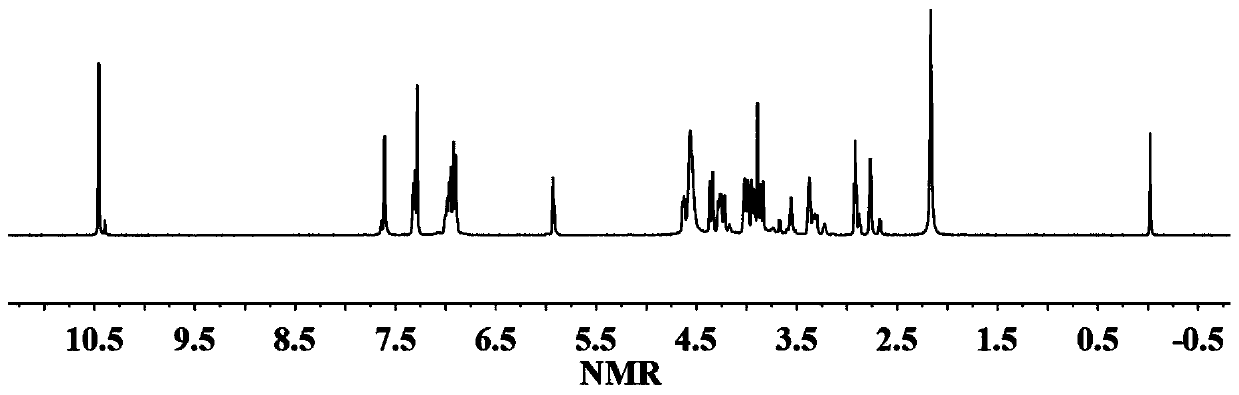
[0036] The proton nuclear magnetic resonance spectrum of th...
Embodiment 2
[0040] (1) Dissolve 1mol of salicylaldehyde, 0.2mol of paraformaldehyde, and 0.01mol of sulfuric acid in 220mL of anhydrous acetic acid, react at 70°C for 35 hours, remove the solvent by rotary evaporation under reduced pressure, wash with water and dry to obtain dihydrate Poplaric aldehyde compound, the productive rate is 81%;
[0041](2) React 1 mol of disalicylaldehyde compound and 5 mol of epichlorohydrin in the presence of 0.5 mol of tetrabutylammonium bromide at 100°C for 6 hours, then remove the solvent by rotary evaporation under reduced pressure, wash with water and dry The epoxidized disalicylaldehyde compound was obtained with a yield of 97%.
[0042] The epoxidized disalicylaldehyde compound obtained and the curing agent m-phenylenediamine are mixed uniformly according to the molar ratio of epoxy and amino group one to two and aldehyde group and amino group one to one respectively in the blast oven, as follows Temperature program curing: curing at 80°C for 2 hours...
Embodiment 3
[0045] (1) Dissolve 1mol of salicylaldehyde, 0.4mol of paraformaldehyde, and 0.03mol of sulfuric acid in 260mL of anhydrous acetic acid, react at 80°C for 24 hours, remove the solvent by rotary evaporation under reduced pressure, wash with water and dry to obtain dihydrate Poplaric aldehyde compound, the productive rate is 91%;
[0046] (2) React 1 mol of disalicylaldehyde compound and 8 mol of epichlorohydrin in the presence of 0.3 mol of tetrabutylammonium bromide at 90°C for 8 hours, then remove the solvent by rotary evaporation under reduced pressure, wash with water and dry The epoxidized disalicylaldehyde compound was obtained with a yield of 95%.
[0047] Mix the obtained epoxidized disalicylaldehyde compound and curing agent ethylenediamine according to the molar ratio of epoxy and amino group one to two and aldehyde group and amino group one to one respectively, and then heat up in a blast oven as follows Programmed temperature curing: curing at 80°C for 2 hours, 120...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com