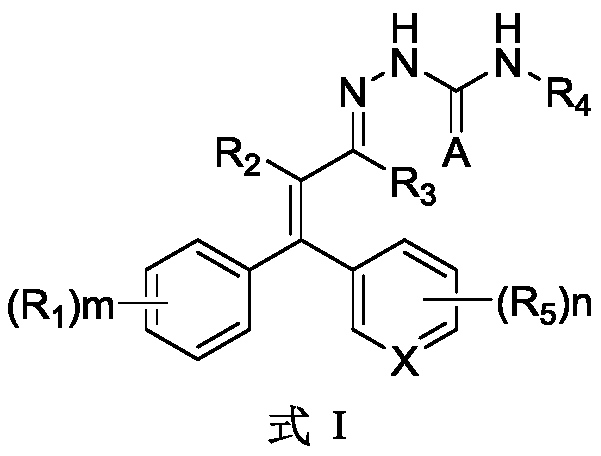
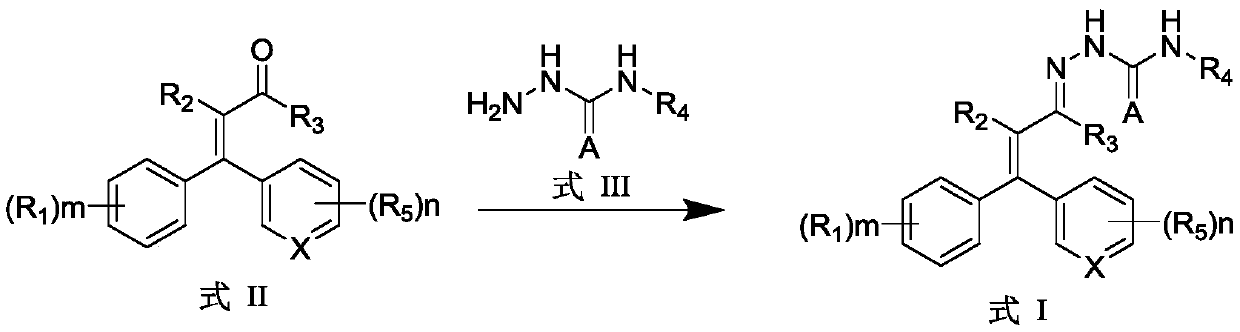
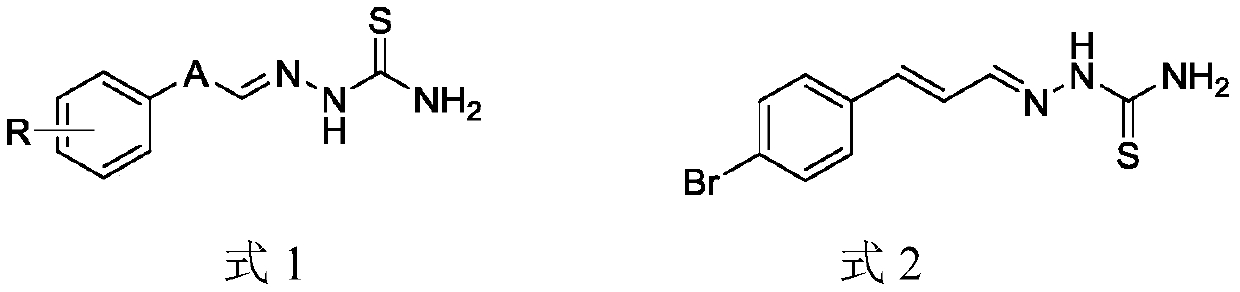
Aryl substituted thiosemicarbazone compound and preparation method and application thereof
A technology of thiosemicarbazones and compounds, which is applied in the field of aryl-substituted thiosemicarbazones and their preparation, and can solve the problem of less thiosemicarbazones
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1, the preparation of aryl-substituted thiosemicarbazone compound I-07
[0049]
[0050] Weigh aldehyde (0.33g, 1.19mmol) and dissolve it in 10mL of absolute ethanol, add thiosemicarbazide (0.11g, 1.19mmol) to the aldehyde, and add 2 drops of glacial acetic acid as a catalyst, stir at room temperature (25°C) until the raw material The reaction was complete (12 hours of reaction). Purified by filtration, washed with ethanol, collected solid, and dried to obtain 0.37 g of white solid. Yield 90.4%. 1H NMR(300MHz,DMSO)δ11.24(s,1H),8.19(s,1H),7.72(d,J=9.8Hz,1H),7.65(s,1H),7.55(d,J=8.4Hz , 2H), 7.44 (d, J = 8.6Hz, 2H), 7.33–7.20 (m, 4H), 6.80 (d, J = 9.8Hz, 1H).
[0051] According to the same method as the above preparation of compound I-07, only R1 and R5 in formula I are replaced with the corresponding substituents as shown in Table 1 to obtain products I-01 ~ I-11, I- 15 to I-20.
Embodiment 2
[0052] Embodiment 2, the preparation of aryl-substituted thiosemicarbazone compound I-14
[0053]
[0054] Aldehyde (0.94g, 2mmol) was dissolved in 10mL of absolute ethanol and added to a 50mL three-necked flask, thiosemicarbazide (0.19g, 2mmol) was added to the reaction solution, 3 drops of glacial acetic acid were added dropwise, and stirred at room temperature (25°C) for 12 Hour. Filtration, 0.95 g of white solid after drying, yield 88%. 1H NMR(300MHz,DMSO)δ11.16(s,1H),8.09(s,1H),7.72(d,J=9.8Hz,1H),7.54(s,1H),7.19–7.11(m,2H) ,7.09–7.01(m,2H),6.94–6.86(m,2H),6.85–6.77(m,2H),6.59(d,J=9.8Hz,1H),0.94(d,18H),0.20(d ,12H).
Embodiment 3
[0055] Embodiment 3, the preparation of compound 1-12
[0056]
[0057] Preparation of Intermediate 1:
[0058] Dissolve TBS aldehyde (1.11 g, 2.36 mmol) in 10 mL of tetrahydrofuran, and stir in an ice-salt bath. Tetrabutylammonium fluoride (5 mL, 5 mmol, 1 M) was added dropwise to the reaction solution in an ice-salt bath. With the addition of tetrabutylammonium fluoride, the reaction solution began to turn black, and finally the color turned brownish yellow. React in an ice bath for 2 hours, add 7 mL of water for post-treatment, then add 10 mL of 10% aqueous sodium bicarbonate solution, extract with a separatory funnel, and collect chloroform (3×40 mL) to collect the organic phase. After desolvation under reduced pressure, it was purified by column using ethyl acetate as the eluent to obtain 0.52 g of yellow liquid with a yield of 100%.
[0059] Preparation of target compound I-12:
[0060] Intermediate 1 (0.52g, 2.36mmol) was dissolved in 5mL ethanol and added to a 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com