High-efficiency thick-film full-polymer solar cell active layer material as well as preparation method and application thereof
A solar cell, high-efficiency technology, applied in circuits, photovoltaic power generation, electrical components, etc., to achieve high efficiency, reduce production costs, and simplify device preparation processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
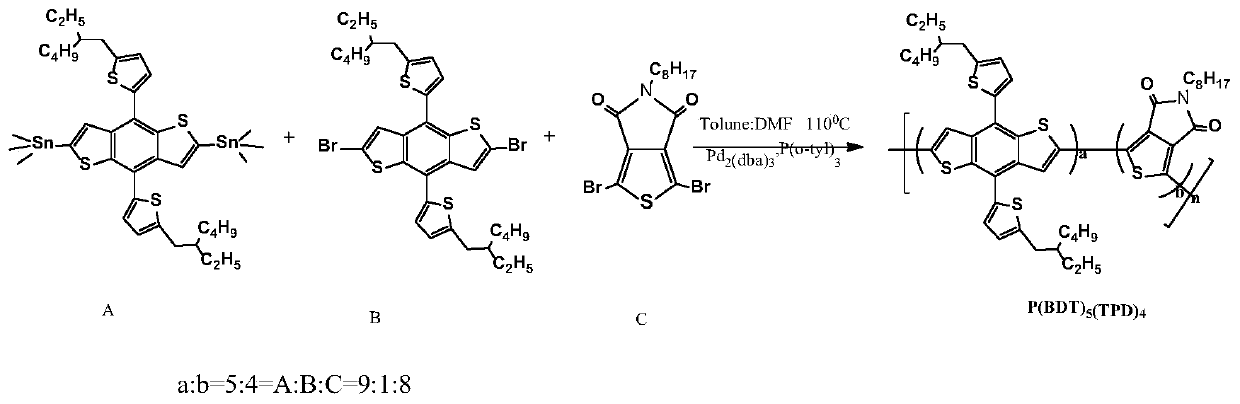
[0038] Polymer Donor P(BDT)5 (TPD) 4 Synthesis
[0039] chemical reaction flow chart figure 1 Shown, concrete reaction steps and reaction conditions are as follows:
[0040] Under the protection of nitrogen, the monomer A (2,6-bis(trimethyltin)-4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-b :4,5-b']dithiophene) (0.110mmol) monomer B (2,6-dibromo-4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzo[ 1,2-b:4,5-b']dithiophene) (0.012mmol) monomer C (1,3-dibromo-5-octyl-4H-thieno[3,4-c]pyrrole-4 , 6(5H)-diketone) (0.098mmol), catalyst tris(dibenzylideneacetone)dipalladium (Pd 2 (dba) 3 ) (5mol%) and the ligand tris(o-methylphenyl)phosphine (P(o-tyl) 3 ) (10mol%) was mixed and dissolved in 3mL of a mixed solvent of toluene and N,N dimethylformamide (5:1). The reaction solution was stirred and refluxed at 110°C for 48 hours, then capped with phenylboronic acid (0.11 mmol), and continued for 12 hours; then 0.3-0.5 mL of bromobenzene was added for capping, reacted at 110°C for 12 hou...
Embodiment 2
[0046] Polymer Donor P(BDT) 2 (TPD) 1 Synthesis
[0047] chemical reaction flow chart figure 2 Shown, concrete reaction steps and reaction conditions are as follows:
[0048] Under the protection of nitrogen, the monomer A (2,6-bis(trimethyltin)-4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-b :4,5-b']dithiophene) (0.110mmol) monomer B (2,6-dibromo-4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzo[ 1,2-b:4,5-b']dithiophene) (0.037mmol) monomer C (1,3-dibromo-5-octyl-4H-thieno[3,4-c]pyrrole-4 , 6(5H)-diketone) (0.074mmol), catalyst tris(dibenzylideneacetone)dipalladium (Pd 2 (dba) 3 ) (5mol%) and the ligand tris(o-methylphenyl)phosphine (P(o-tyl) 3 ) (10mol%) was mixed and dissolved in 3mL of a mixed solvent of toluene and N,N dimethylformamide (5:1). The reaction solution was stirred and refluxed at 110°C for 48 hours, then capped with phenylboronic acid (0.11 mmol), and continued for 12 hours; then 0.3-0.5 mL of bromobenzene was added for capping, reacted at 110°C for 12 ...
Embodiment 3
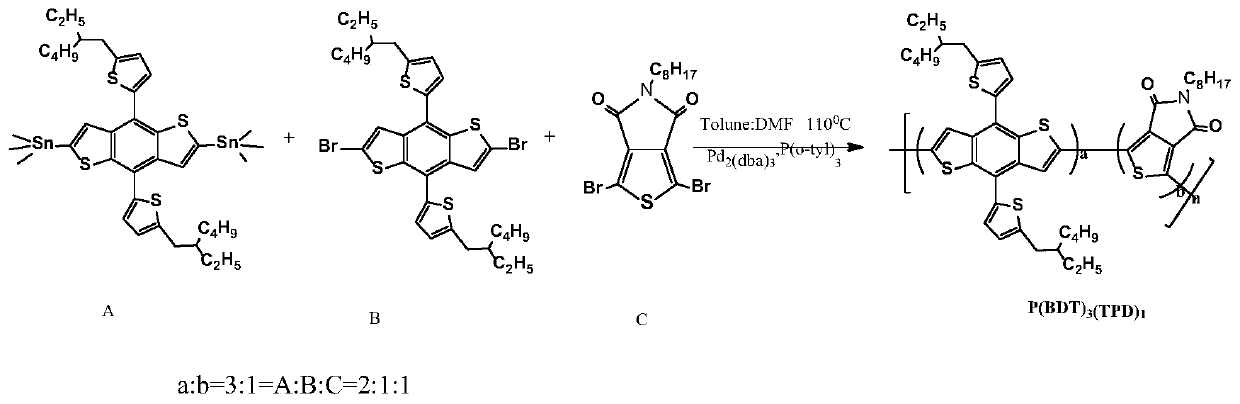
[0054] Polymer Donor P(BDT) 3 (TPD) 1 Synthesis
[0055] chemical reaction flow chart image 3 Shown, concrete reaction steps and reaction conditions are as follows:
[0056] Under the protection of nitrogen, the monomer A (2,6-bis(trimethyltin)-4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-b :4,5-b']dithiophene) (0.110mmol) monomer B (2,6-dibromo-4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzo[ 1,2-b:4,5-b']dithiophene) (0.055mmol) monomer C (1,3-dibromo-5-octyl-4H-thieno[3,4-c]pyrrole-4 , 6(5H)-diketone) (0.055mmol), catalyst tris(dibenzylideneacetone)dipalladium (Pd 2 (dba) 3 ) (5mol%) and the ligand tris(o-methylphenyl)phosphine (P(o-tyl) 3 ) (10mol%) was mixed and dissolved in 3mL of a mixed solvent of toluene and N,N dimethylformamide (5:1). The reaction solution was stirred and refluxed at 110°C for 48 hours, then capped with phenylboronic acid (0.11 mmol), and continued for 12 hours; then 0.3-0.5 mL of bromobenzene was added for capping, reacted at 110°C for 12 h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com