Immunostimulant
An immunostimulatory and immunostimulatory technology, applied in the field of immunostimulants, can solve the problems of no research, changes, unknown adsorption capacity of purified tuberculin, etc., and achieve the effect of preventing recurrence or metastasis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0124] Hereinafter, the present invention will be described in more detail by way of examples, but the scope of the present invention is not limited to the following examples.
[0125] The terms and concepts in the present invention are based on the meanings of the terms commonly used in the art, and the techniques used in order to implement the present invention can be easily and accurately understood by those skilled in the art based on well-known documents, etc. implemented. In addition, various analyses etc. were performed using the methods described in the used analytical instruments, reagents, operating instructions of the kits, catalogues, and the like.
[0126] [Table 1]
[0127] List of acronyms
[0128]
[0129]
example 1
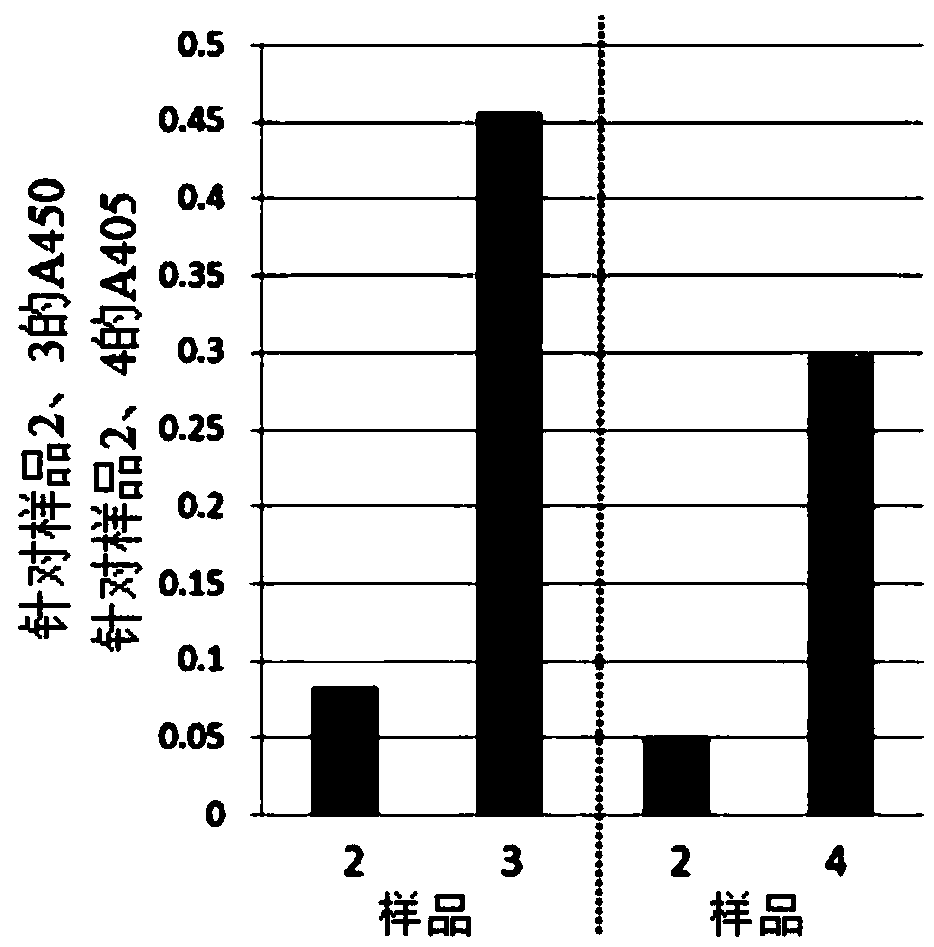
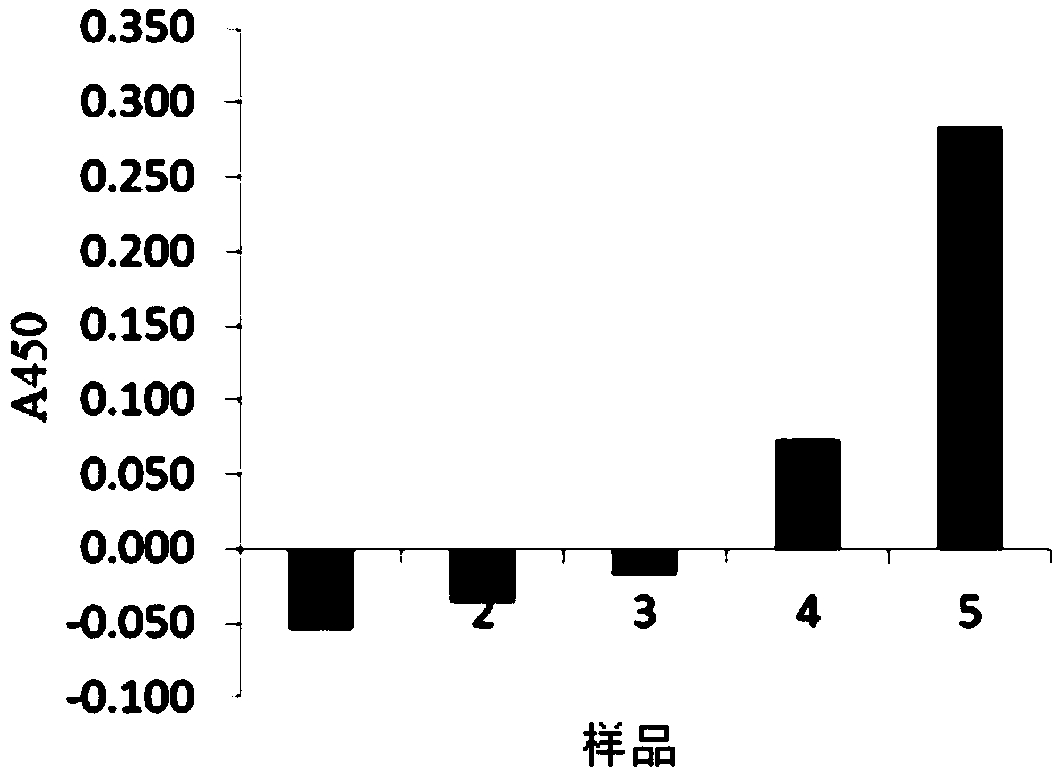
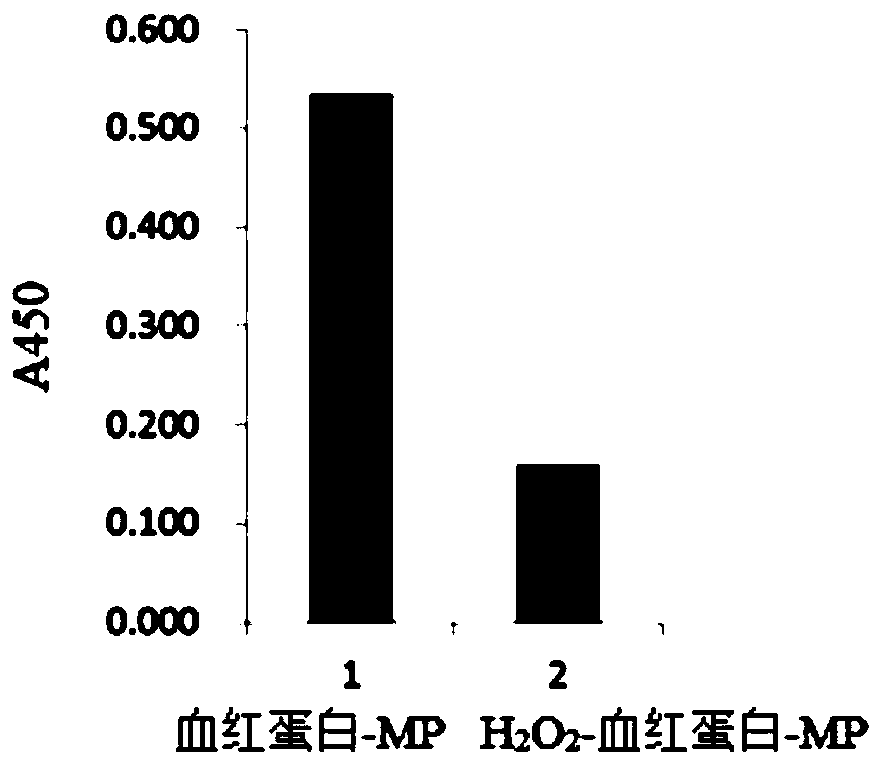
[0130] Example 1: Preparation of Carrier Containing Denatured Coagulated Solid Plasma, and Stimulation Effect of Antigen-presenting Cells Using Undenatured Hemoglobin Adsorption Complex or Undenatured Myoglobin Adsorption Complex
[0131] It is known that if the human macrophage-like cell line THP-1 is induced to differentiate under the culture of Phorbol 12-Myristate 13-Acetate (phorbol 12-myristate 13-acetate, PMA), not only It exhibits phagocytic ability (Non-Patent Document 18), and also acquires antigen-presenting ability to become antigen-presenting cells (Non-Patent Document 19). Furthermore, when the cells are pretreated with human interferon gamma (IFNg) as a cytokine, the antigen-presenting ability to T cells is enhanced (Non-Patent Document 20). Differentiated and phagocytosed THP-1 cells produce TNFα (Non-Patent Document 21). It is known that the production of TNFα shows the activation of macrophages (or antigen-presenting cells), and the activation of macrophages (...
example 2
[0148] Example 2: Stimulatory effect of antigen-presenting cells by denaturation and coagulation of solidified albumin / hemoglobin complex
[0149] In Example 1, it was shown that the substance in which undenatured hemoglobin is adsorbed on the denatured coagulated solid plasma carrier has a stimulating effect on antigen-presenting cells, but albumin, which is a representative example of serum proteins, and erythrocyte-derived hemoglobin were mixed in advance. , to study the stimulating activity of antigen-presenting cells when the mixture was denatured, coagulated and solidified.
[0150] (1) Preparation method of sample for bioassay
[0151] For a commercially available biological preparation standard, human serum albumin (manufactured by the Institute of Chemistry and Serotherapy, donated blood albumin 25 "Kaxueken", hereinafter referred to as "25% HSA") was prepared at the ratio shown below A liquid obtained by mixing human hemoglobin (manufactured by SIGMA, H7379-5G, diss...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com