Probe group for detecting pathogenic/susceptible genes of hereditary cardiomyopathy/arrhythmia
A technology for susceptibility genes and cardiomyopathy, which is applied in the fields of genetic engineering and molecular genetics, can solve the problems of difficult diagnosis, time-consuming and labor-intensive detection costs, and lagging progress, and achieve the goal of multiple detection sites, strong practicability, and low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Embodiment 1 Design and preparation of the probe set of the present invention
[0069] 1. Screening of pathogenic and / or susceptibility genes
[0070] The pathogenic and / or susceptibility genes in this example are MYH7, TNNT2, TPM1, MYBPC3, PRKAG2, TNNI3, MYL3, TTN, MYL2, ACTC1, CSRP3, TNNC1, MYH6, VCL, MYOZ2, JPH2, PLN, CALR3, NEXN, MYPN, ACTN2, LDB3, TCAP, FLNC, MYLK2, CAV3, FHL1, CRYAB, TRIM63, ANKRD1, KLF10, MYOM1, FXN, CASQ2, PDLIM3, MYO6, ACTA1, FHOD3, ELAC2, MRPL3, TRMU, DES, MAP2K1, MAP2K2, GLA, CALM3, BRAF, NDUFAF1, SLC25A3, SLC25A4, NDUFV2, RAF1, SOS1, MTO1, SRI, OBSCN, TACO1, FASTKD2, COX20, COX10, PET100, APOPT1, COX14, COX6B1, AGK, MGME1, GAA, AGL, IDS, PTPN11, KRAS, NRAS, RIT1, SOS2, LZTR1, CBL, SHOC2, NF1, LAMP2, NSD1, CDKN1C, H19, KCNQ1OT1, MRPS16, TSFM, MRPS22, AARS2, MRPL44, GTPBP3, SCO2, COX15, COA5, COA6, ACAD9, MLYCD, SLC22A5, FHL2, LMNA, SCN5A, EYA4, SGCD, ABCC9, TMPO, PSEN1, PSEN2, FKTN, DSG2, RBM20, SDHA, BAG3, LAMA4, PRDM16, GATAD1, DMD, DSP, TN...
Embodiment 2
[0077] Embodiment 2 The composition, preparation and application of the kit of the present invention.
[0078] The kit for detecting the pathogenic and / or susceptibility genes of cardiomyopathy described in this embodiment is to carry out the molecular genetic diagnosis of the individual by detecting the mutation of the above-mentioned 312 pathogenic and / or susceptibility genes Or a kit for morbidity risk prediction.
[0079] 1. The composition of the kit
[0080] The components contained in the kit are: the probe set obtained in Example 1 (160 μL, 150 ng / μL), enrichment buffer (208 μL), hybridization buffer (800 μL), binding buffer (3.2 mL), washing Solution 1 (9mL), rinse solution 2 (45mL), NaOH solution (0.1M, 1mL), Tris-HCl buffer (1M, pH 7.5, 1.2mL), PCR reaction solution (580μL), TE buffer (800μL, 10mM Tris-HCl, 1mM EDTA, adjust the pH to 8.0, and adjust the volume to 500mL with water). Wherein each buffer composition is as follows:
[0081] (1) Enrichment buffer (pe...
Embodiment 3
[0135] Example 3 Clinical diagnosis of the probe set of the present invention
[0136] Molecular genetic diagnosis of 8 patients with hereditary cardiomyopathy and related syndromes using the probe set prepared in Example 1 of the present invention. The patients were clinically diagnosed cardiomyopathy-related diseases in the Department of Cardiovascular Medicine of the hospital. Each patient signed an informed and voluntary agreement, which was approved by the hospital's medical ethics committee.
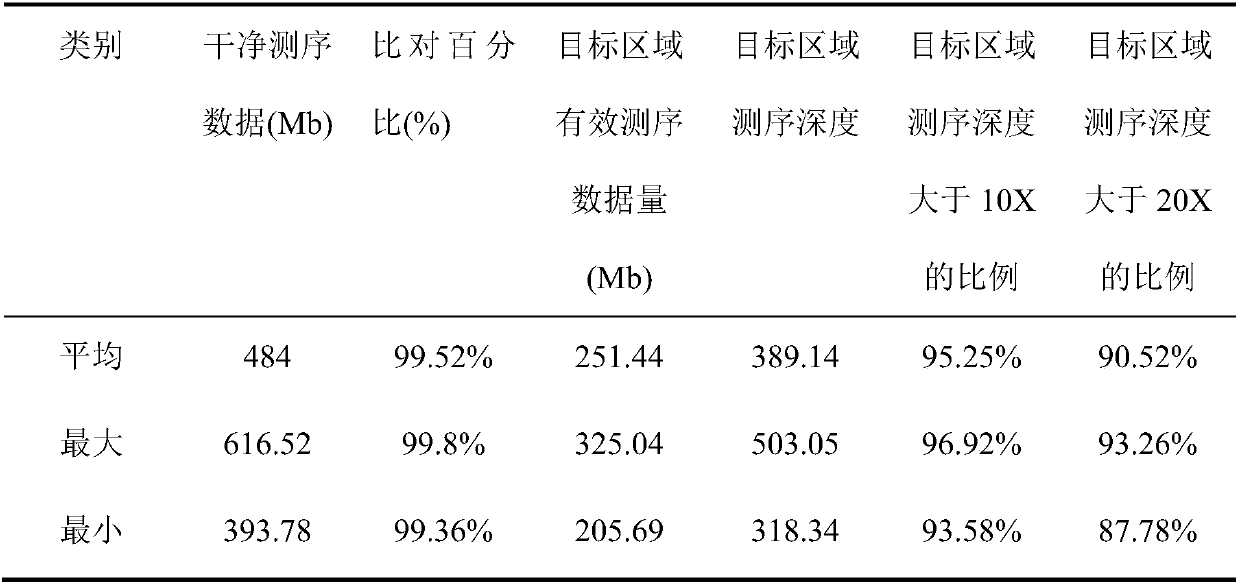
[0137] The molecular genetic diagnosis of the patient in this example was performed according to the composition, preparation and application steps of the kit described in Example 2. The results of molecular genetic diagnosis are shown in Table 2.
[0138] Table 2 Molecular genetic diagnoses made in patients with hereditary cardiomyopathy and related syndromes
[0139]
[0140]
[0141] 1. Molecular genetic diagnosis of a patient with glycogen storage disease
[0142]The c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com