Modeling method for adverse reactions of digestive tract after chemotherapy, model and application
A technology for model establishment and adverse reactions, which can be used in medical preparations containing active ingredients, pharmaceutical formulations, compound screening/testing, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0043] Experimental Drugs:
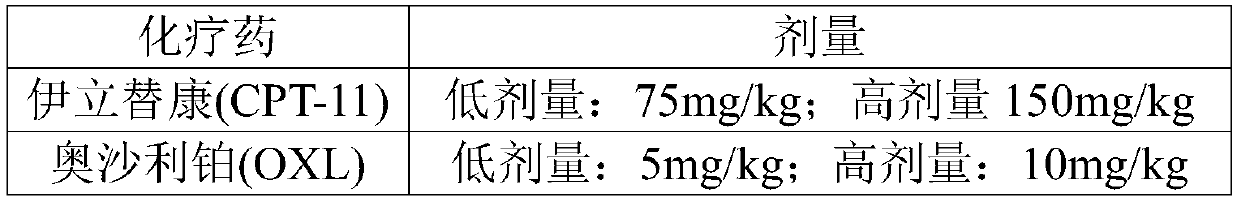
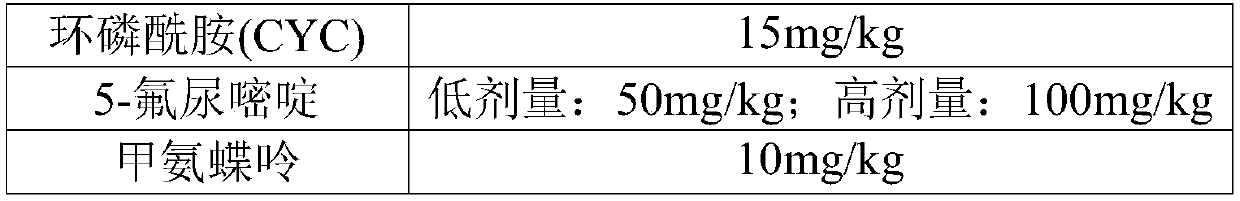
[0044] Oxaliplatin (50mg / bottle) (Jiangsu Hengrui Medicine Co., Ltd.), 5-fluorouracil (100mg / 10ml) (Jiangsu Hengrui Medicine Co., Ltd.), irinotecan hydrochloride for injection (40mg / bottle) (Jiangsu Hengrui Medicine Co., Ltd.) Hengrui Medicine Co., Ltd.), cyclophosphamide (20mg / bottle), methotrexate (50mg / bottle).
[0045] Dosing regimen:
[0046] The CT tumor-bearing BALB / c mice were divided into nine groups, and chemotherapy drugs such as irinotecan, oxaliplatin, 5-fluorouracil, cyclophosphamide and methotrexate were selected, and the chemotherapy drugs were injected intraperitoneally except for the blank group. Administer once a day, observe the survival status and defecation of the mice during the administration process, and finally evaluate the intestinal toxicity of the tumor-bearing mice caused by various chemotherapeutic drugs based on the survival rate and intestinal propulsion rate of the mice in each group ten days later. Administratio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com