A kit for detecting pathological myopia and its use method and application
A kit and pathological technology, applied in the field of kits for detecting pathological myopia, can solve the problems of increasing eyesight and not being able to effectively control the development of myopia, and achieve strong specificity, which is conducive to the development of new drugs and has a good market application prospect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
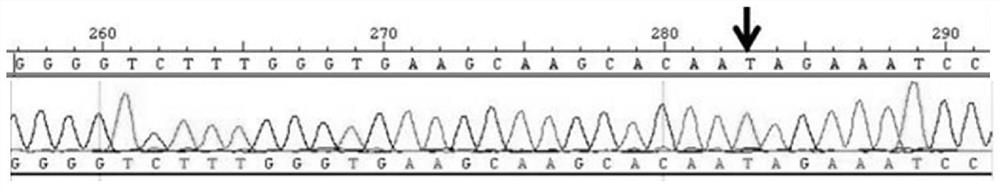
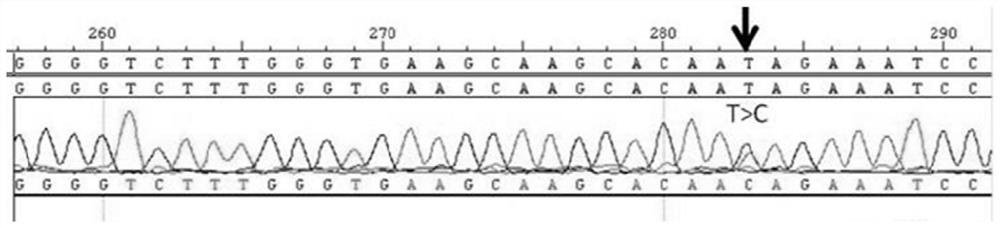
[0032] KIAA1462 Detection of Gene Mutations
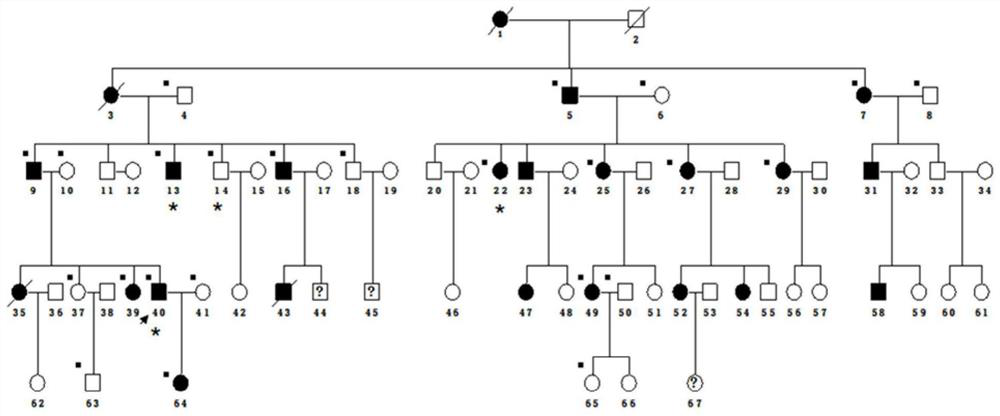
[0033] An autosomal dominant 5-generation pedigree strictly followed the diagnostic criteria: ① myopia ≤ -6.0D; ② characteristic fundus changes including optic disc arc spot, leopard pattern fundus, macular degeneration and posterior staphyloma; ③ The age of onset is before elementary school; ④ myopia progressively deepening; Myopia, that is, patients diagnosed as pathological myopia; ①② is a necessary condition. After the phenotype was determined, the family survey was collected. Genealogy maps were generated using Cyrillic software.
[0034] Collect the peripheral blood of at least three patients and one normal immediate relative in the family, extract genomic DNA, use the Agilent Human All Exon Kit exome capture chip, prepare a cluster for data processing, and then find three patients through bioinformatics analysis The total number of mutations in the database is about 2-5 thousand, and the public database is used to filter...
Embodiment 2
[0037] A kit for detecting pathological myopia of the present invention, the kit includes KIAA1462 A reagent for the gene fragment at the c.3385th gene site of the third exon of the gene; the reagent includes a primer pair, the sequence of the sense primer of the primer pair is: TGGGCGAGATAGCAACTCTT, and the sequence of the antisense primer of the primer pair is: GAGGTTAGCAACGGAATGGA. The reagent also includes buffer, mixed solution, magnesium chloride solution, Taq enzyme with a concentration of 1U / μL, DNA template with a concentration of 30ng / μL and double distilled water, the buffer is PCR Buffer, and the mixed solution is dNTP mixture. Wherein, each component and dosage in the reagent are shown in Table 1.
[0038] Table 1 Composition of reagents
[0039] serial number components Dosage 1 10×PCR Buffer 5μL 2 dNTP mixture 2mL each 5μL 3 2.5mL MgCl 2
Embodiment 3
[0041] A method for using a test kit for detecting pathological myopia of the present invention comprises the following steps:
[0042] 1) Genomic DNA extraction: Use the QIAamp DNA Blood Mini Kit kit from QIAGEN to extract peripheral blood genomic DNA; dissolve the obtained DNA in TE for storage, correct the DNA working concentration to 30ng / μL, and store in a -20°C refrigerator;
[0043] 2) PCR amplification: take the kit, the composition of 25 μL PCR reaction system is shown in Table 1, the sequence of the sense primer is: TGGGCGAGATAGCAACTCTT, the sequence of the antisense primer of the primer pair is: GAGGTTAGCAACGGAATGGA; the conditions for PCR amplification are: 95 ℃, 5min; 95℃, 30s, 65℃, 45s, 72℃, 45s, 35 cycles; 72℃, 10min; 4℃, keep warm to get the PCR product;
[0044] 3) PCR product purification: take 5 μL of the PCR product obtained in step 2), add 1 μL of ExoSAP-IT (US PCR purification reagent) from USB Company, and perform purification reaction. The conditions of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com