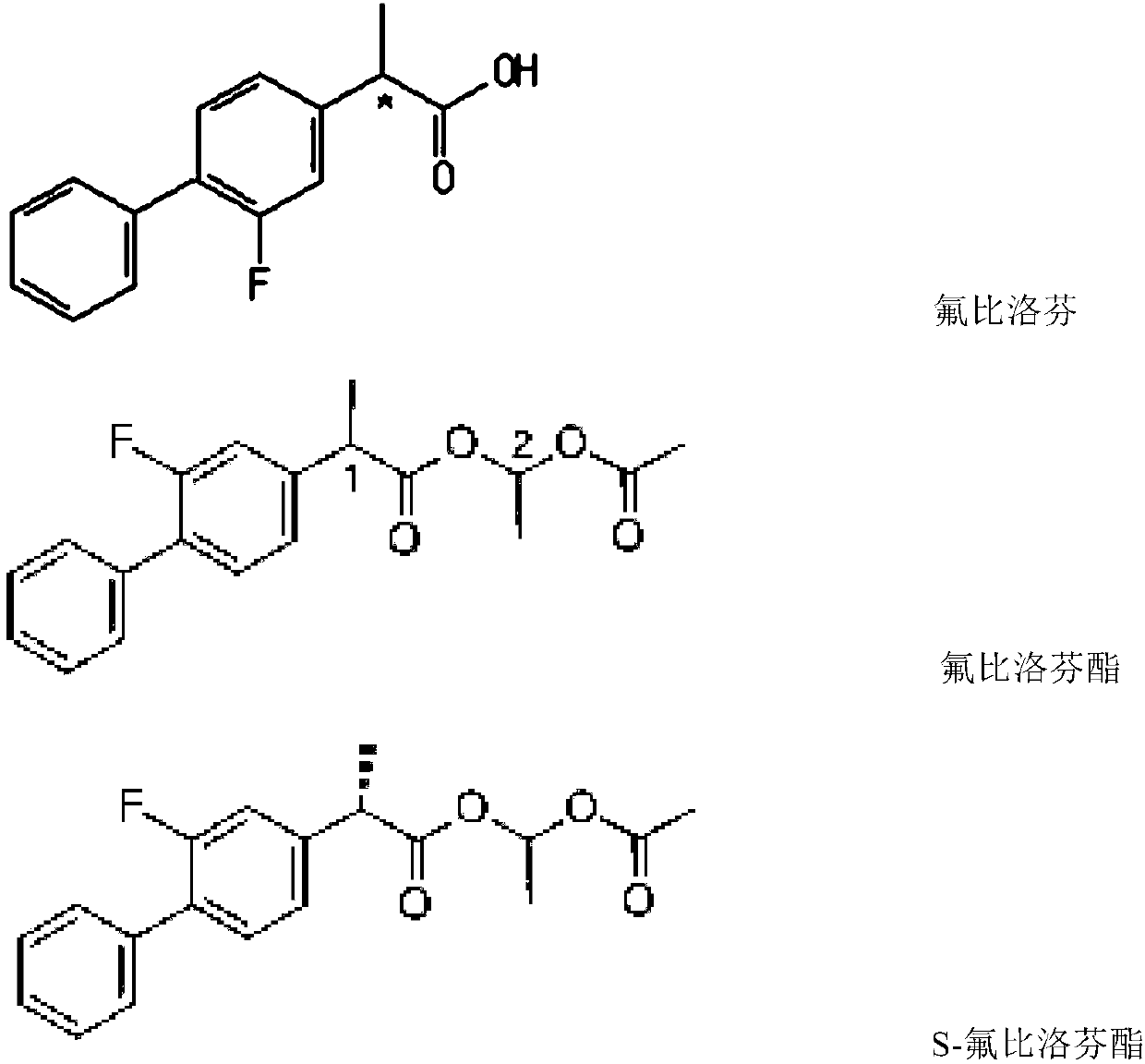
Method for resolving and determining flurbiprofen axetil and S-flurbiprofen axetil
A technology of flurbiprofen axetil and degree of separation, applied in the field of medicine, can solve the problems of lack of cyclooxygenase inhibitory activity, increased gastrointestinal side effects and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] The present invention will be further illustrated by specific preparation examples and test examples below, but it should be understood that these examples are only used for more detailed description and should not be construed as limiting the present invention in any form.
[0055] The present invention provides general and / or specific descriptions of the materials and test methods used in the tests. While many of the materials and methods of manipulation which are employed for the purposes of the invention are well known in the art, the invention has been described here in as much detail as possible. It will be clear to those skilled in the art that in the following, unless otherwise specified, the materials and operation methods used in the present invention are well known in the art.
[0056] 1. Supply of flurbiprofen axetil and S-flurbiprofen axetil
[0057] Flurbiprofen axetil is a commercially available bulk drug (the product approved by the State Food and Dru...
Embodiment 1
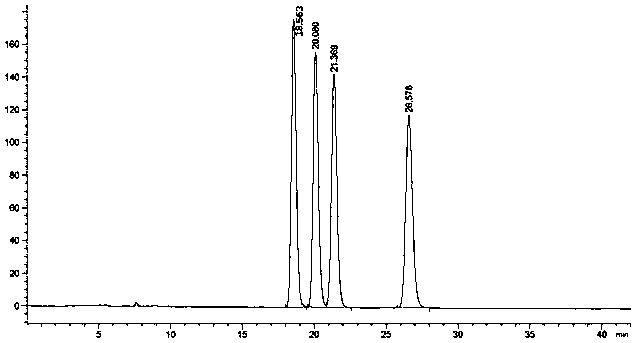
[0061] The HPLC resolution and determination of embodiment 1, flurbiprofen axetil and S-flurbiprofen axetil
[0062] (1) Dosing:
[0063] Accurately weigh flurbiprofen axetil or S-flurbiprofen axetil need testing 10mg, put in 10ml measuring bottle, add mixed solvent to dissolve and dilute to scale, shake up, as need testing solution;
[0064] Draw need testing solution 1ml and put in 100ml measuring bottle, add mixed solvent and dilute to scale, shake up, as contrast solution (its concentration is equivalent to 1% of need testing solution);
[0065] In addition, accurately weigh 10mg of flurbiprofen axetil racemate reference substance, put it in a 100ml measuring bottle, add a mixed solvent to dissolve and dilute to the mark, shake well, and make a solution containing 0.1mg per 1ml, as a system suitability test solution ;
[0066] (2) Chromatographic conditions and system suitability test:
[0067] A chromatographic column using starch-tris(3,5-dimethylphenylcarbamate) as...
Embodiment 2
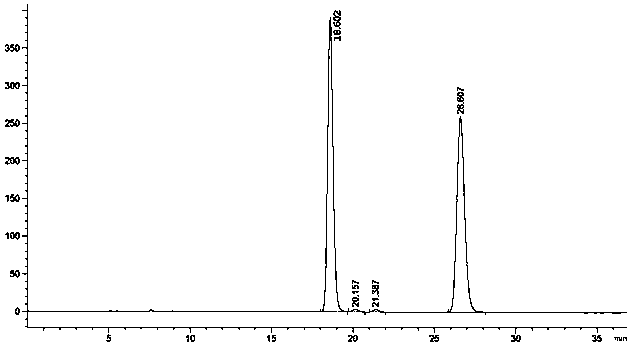
[0074] The HPLC resolution and determination of embodiment 2, flurbiprofen axetil and S-flurbiprofen axetil
[0075] (1) Dosing:
[0076] Accurately take flurbiprofen axetil need testing sample or S-flurbiprofen axetil need testing sample amount, put in 10ml measuring bottle, add mixed solvent to dissolve and dilute to scale, shake up, as need testing solution (0.01mg / ml);
[0077] Draw 0.2ml of the test solution, put it in a 100ml measuring bottle, add a mixed solvent to dilute to the mark, shake well, and use it as a control solution;
[0078] In addition, accurately weigh an appropriate amount of flurbiprofen axetil racemate reference substance, put it in a 10ml measuring bottle, add mixed solvent to dissolve and dilute to the mark, shake well, and make a solution with the same concentration as the test solution, as the system suitability test solution;
[0079] (2) Chromatographic conditions and system suitability test:
[0080] A chromatographic column using starch...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com