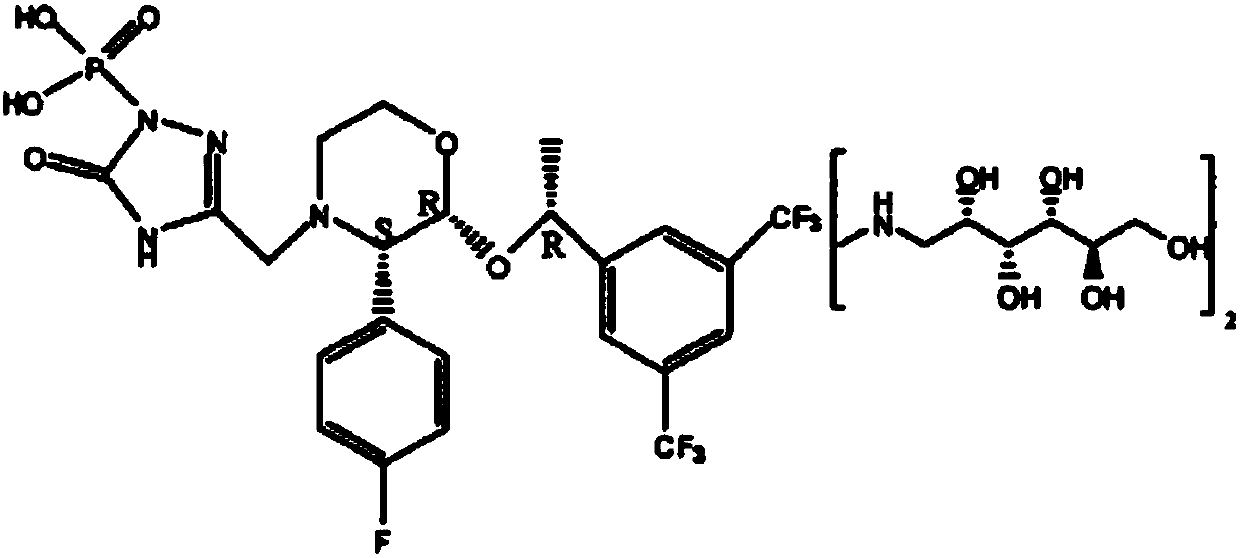
Preparation method of fosaprepitant freeze-dried powder injection
A technology of fosaprepitant and freeze-drying protective agent, which is applied in the field of medicine and can solve problems such as the development of fosaprepitant slow-control reagent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
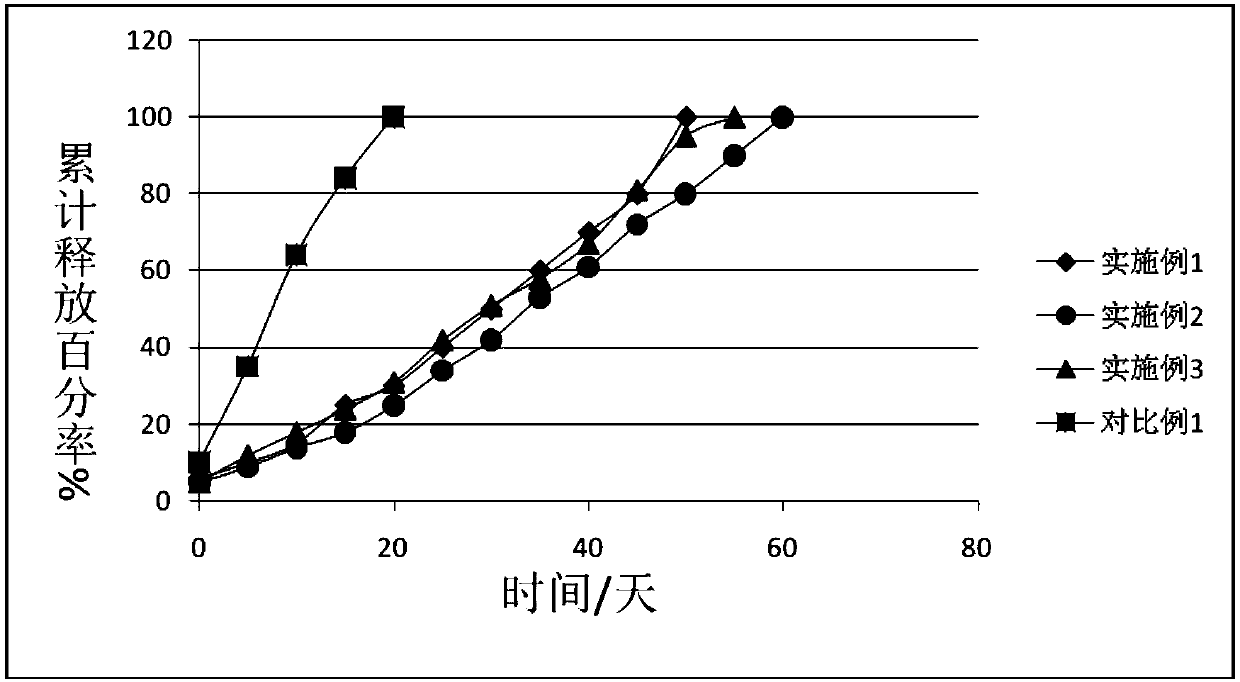
Embodiment 1
[0038] A preparation method of fosaprepitant freeze-dried powder injection, is characterized in that, the step comprises:
[0039] (1) Preparation of oil phase: First, 1 part of fosaprepitant and 3 parts of PLGA are added to 30 parts of acetone for dissolution, and the acetone is removed by rotary evaporation, then 10 parts of soybean oil for injection and 0.2 part of soybean phosphatidylcholine are added and stirred Mix to obtain material 1;
[0040] (2) Water phase preparation: add 1 part of isotonic regulator glycerin and 5 parts of xylitol to 500 parts of water for injection and mix evenly to obtain material 2;
[0041] (3) Mix the material 1 obtained in step (1) and the material 2 obtained in step (2) at 45° C., and mechanically stir at 2000 rpm for 30 minutes to obtain colostrum;
[0042] (4) Distill the colostrum obtained in step (3) with water for injection, adjust the pH to 6 with a pH regulator hydrochloric acid or sodium hydroxide, and circulate and homogeneously e...
Embodiment 2
[0046] A preparation method of fosaprepitant freeze-dried powder injection, is characterized in that, the step comprises:
[0047] (1) Preparation of the oil phase: First, 2 parts of fosaprepitant and 5 parts of PLGA were added to 50 parts of acetone for dissolution, and the acetone was removed by rotary evaporation, and then 20 parts of soybean oil for injection and 1 part of emulsifier egg yolk phosphatidylcholine were added. Alkali is stirred and mixed to obtain material 1;
[0048] (2) Water phase preparation: add 2 parts of glycerin, an isotonic regulator, and 8 parts of xylitol, a freeze-drying protective agent, into 800 parts of water for injection and mix evenly to obtain material 2;
[0049] (3) Mix the material 1 obtained in step (1) and the material 2 obtained in step (2) at 60° C., and mechanically stir at 2500 rpm for 40 minutes to obtain colostrum;
[0050] (4) The colostrum obtained in step (3) is fixed to volume with water for injection, the pH is adjusted to ...
Embodiment 3
[0054] A preparation method of fosaprepitant freeze-dried powder injection, is characterized in that, the step comprises:
[0055] (1) Preparation of the oil phase: First, 3 parts of fosaprepitant and 10 parts of PLGA were added to 60 parts of acetone for dissolution, and the acetone was removed by rotary evaporation, then 30 parts of soybean oil for injection and 2 parts of emulsifier egg yolk phosphatidylcholine were added. Alkali is stirred and mixed to obtain material 1;
[0056] (2) Water phase preparation: add 2 parts of isotonicity regulator sodium chloride and 10 parts of freeze-drying protective agent trehalose into 1000 parts of water for injection and mix evenly to obtain material 2;
[0057] (3) Mix the material 1 obtained in step (1) and the material 2 obtained in step (2) at 85° C., and mechanically stir at 3000 rpm for 30-40 minutes to obtain colostrum;
[0058] (4) Distill the colostrum obtained in step (3) with water for injection, adjust the pH to 9 with a p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com