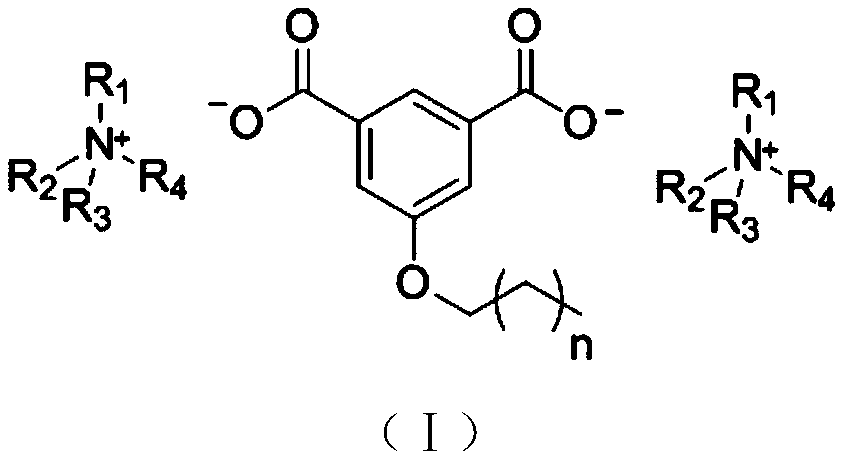
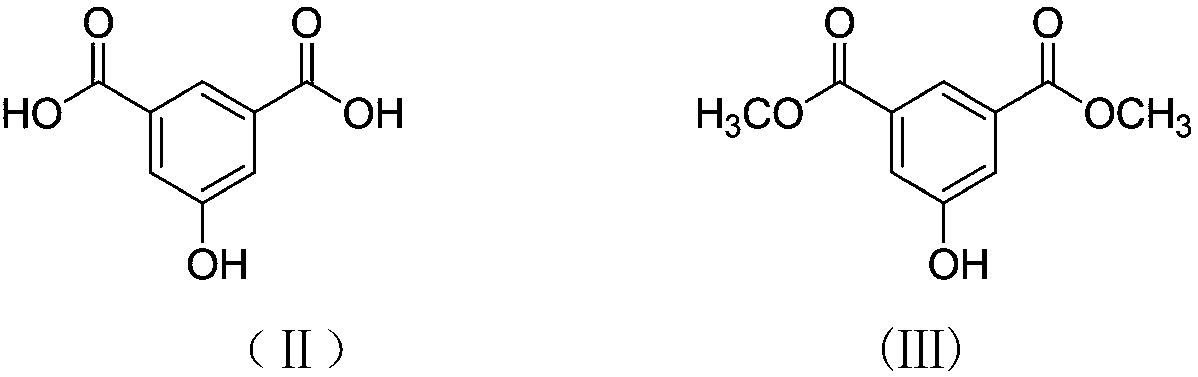Aromatic dicarboxylic acid ionic liquid, as well as preparation method and application thereof
An aromatic dicarboxylic acid and ionic liquid technology, applied in the chemical industry, can solve problems such as unsatisfactory thermal stability, and achieve ideal thermal stability, excellent anti-friction and anti-wear properties.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: Preparation of 5-butoxy-1,3-isophthalic acid diquaternary ammonium salt
[0031] (1) Weigh 25.9353 g of 5-hydroxy-1,3-benzenedicarboxylic acid, 6.8665 g of p-toluenesulfonic acid, and 89.6000 g of methanol into a round bottom flask. Reflux at 75°C for 24 hours. After the reaction, methanol was distilled off, washed with saturated brine, extracted with ethyl acetate, allowed to stand, separated into layers, and dried. The product, methyl 5-hydroxy-1,3-phthalate, was obtained.
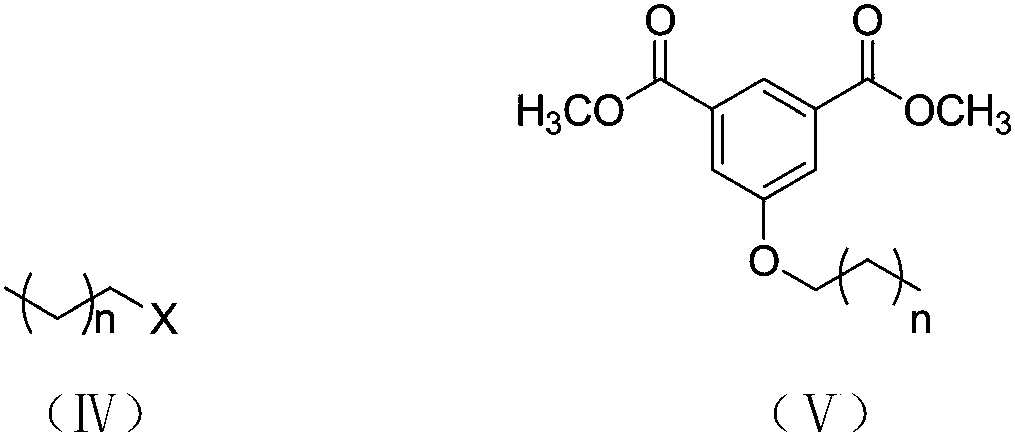
[0032] (2) Methyl 5-hydroxy-1,3-phthalate: Potassium carbonate: 1-bromobutane with a molar ratio of 1:2.5:1.4 Weigh 34.6800g of potassium carbonate and 19.3760g of 1-bromobutane Sequentially add to the flask, add an appropriate amount of N,N-dimethylformamide. Reflux at 85°C for 24 hours. After the reaction, filter off K while hot 2 CO 3 , washed 2 to 3 times with water to remove unreacted K 2 CO 3 . Extract 3 times with petroleum ether, take the organic phase, add anhydrous...
Embodiment 2
[0038] Example 2: Preparation of 5-octyloxy-1,3-isophthalic acid diquaternary ammonium salt
[0039] (1) Weigh 25.9353 g of 5-hydroxy-1,3-benzenedicarboxylic acid, 6.8665 g of p-toluenesulfonic acid, and 89.6000 g of methanol into a round bottom flask. Reflux at 75°C for 24 hours. After the reaction, methanol was distilled off, washed with saturated brine, extracted with ethyl acetate, allowed to stand, separated into layers, and dried. The product, methyl 5-hydroxy-1,3-phthalate, was obtained.
[0040] (2) Methyl 5-hydroxy-1,3-phthalate: Potassium carbonate: 1-bromooctane with a molar ratio of 1:2.5:1.4 Weigh 34.6800g of potassium carbonate and 19.3760g of 1-bromooctane Sequentially add to the flask, add an appropriate amount of N,N-dimethylformamide. Reflux at 85°C for 24 hours. After the reaction, filter off K while hot 2 CO 3 , washed 2 to 3 times with water to remove unreacted K 2 CO 3 . Extract 3 times with petroleum ether, take the organic phase, add anhydrous ...
Embodiment 3
[0046] Example 3: Preparation of 5-dodecyloxy-1,3-isophthalic acid diquaternary ammonium salt
[0047] (1) Weigh 25.9353 g of 5-hydroxy-1,3-benzenedicarboxylic acid, 6.8665 g of p-toluenesulfonic acid, and 89.6000 g of methanol into a round bottom flask. Reflux at 75°C for 24 hours. After the reaction, methanol was distilled off, washed with saturated brine, extracted with ethyl acetate, allowed to stand, separated into layers, and dried. The product, methyl 5-hydroxy-1,3-phthalate, was obtained.
[0048] (2) Methyl 5-hydroxy-1,3-phthalate: Potassium carbonate: 1-bromododecane in a molar ratio of 1:2.5:1.4 Weigh 34.6800g of potassium carbonate, 1-bromododecane 19.3760g was added to the flask in sequence, and an appropriate amount of N,N-dimethylformamide was added. Reflux at 85°C for 24 hours. After the reaction, filter off K while hot 2 CO 3 , washed 2 to 3 times with water to remove unreacted K 2 CO 3 . Extract 3 times with petroleum ether, take the organic phase, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com