Compositions and methods for treatment of her2 positive metastatic breast cancer
A therapeutic composition and composition technology, applied in chemical instruments and methods, drug combinations, medical raw materials derived from mammals, etc., can solve problems such as low toxicity and adverse side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
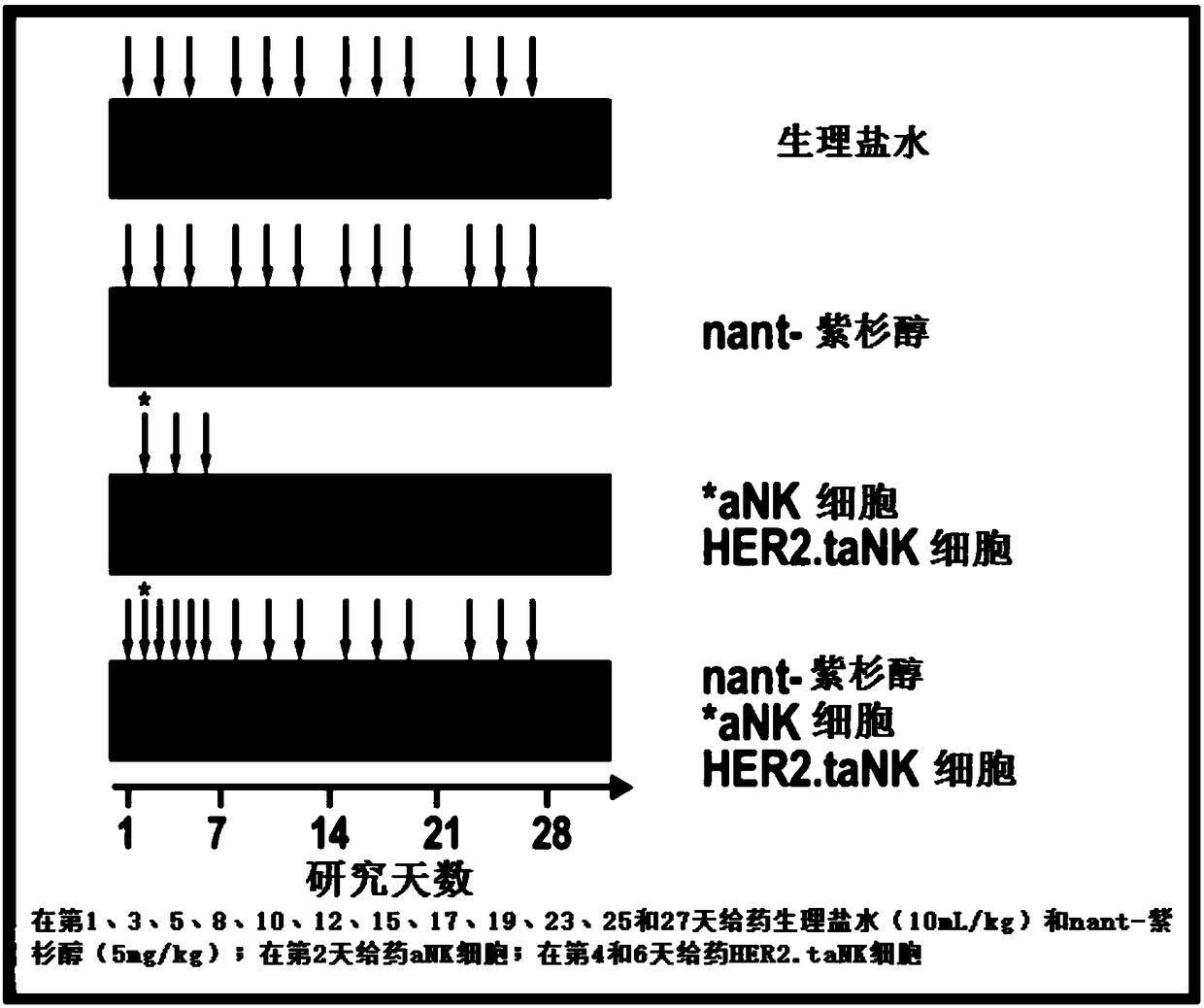
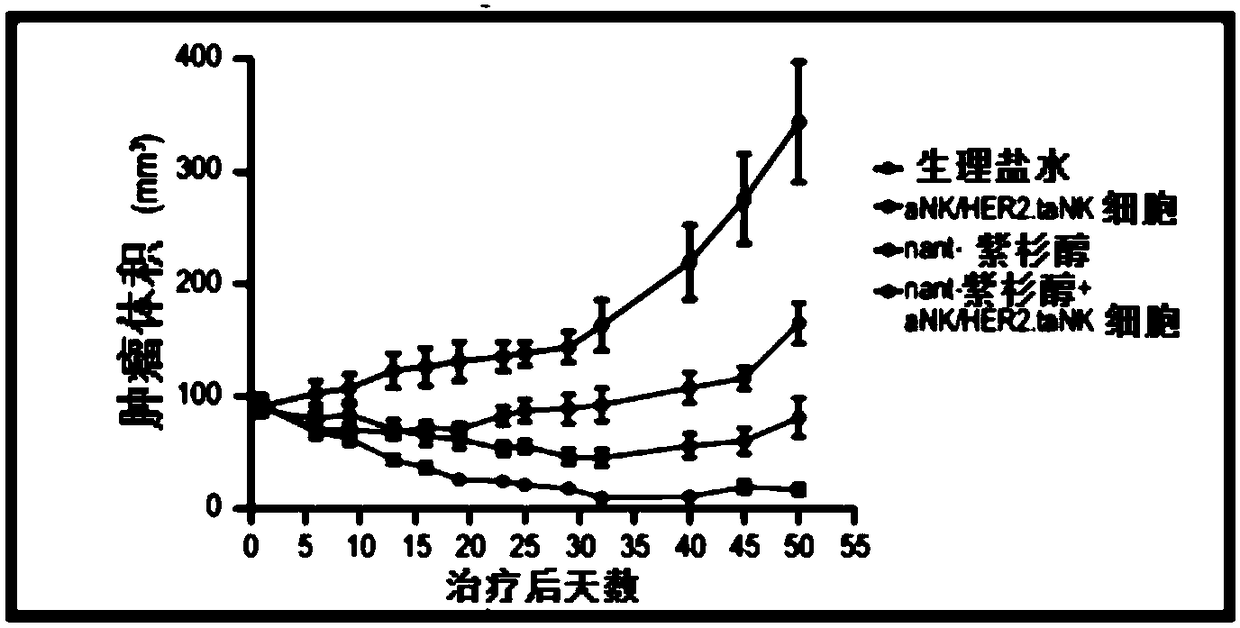
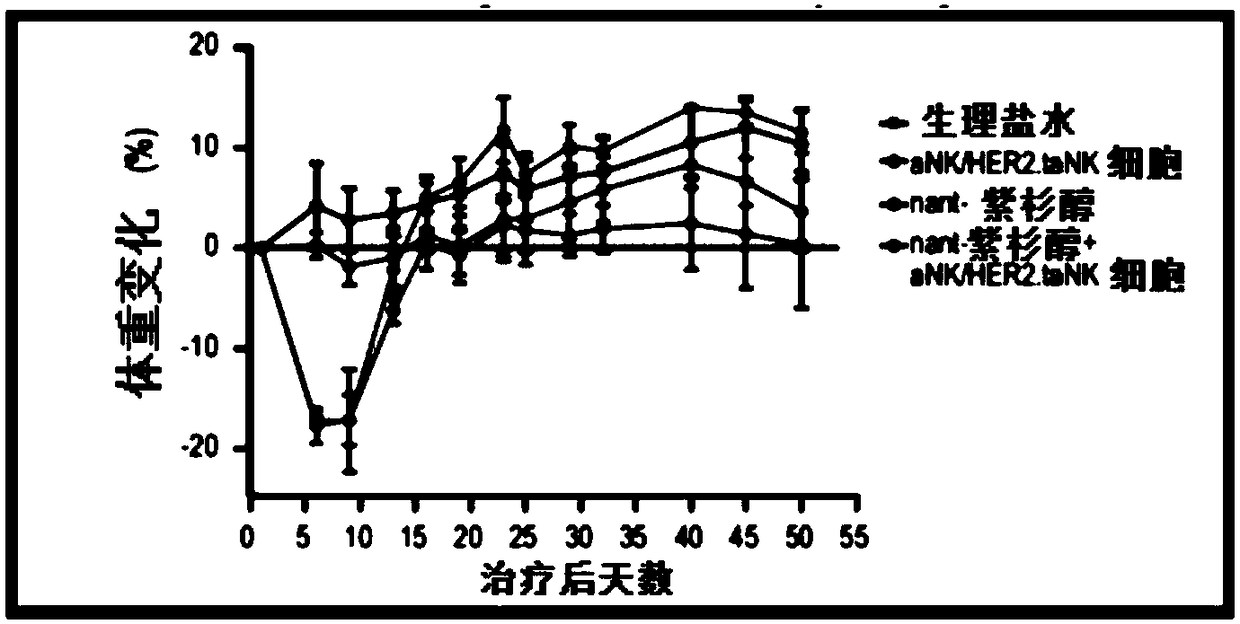
[0054] In an exemplary therapeutic approach to determine the efficacy of HER2.taNK in combination with metronomic Nant-paclitaxel (Abraxane) in a mouse model of HER2-positive breast cancer, the inventors used HER2.taNK cells as previously described (MolTher .2015;23(2):330-338). MDA-MB-453 cells (0.1 mL of 1 × 10 in 50% Matrigel 8 cells / mL) were injected subcutaneously into the left and right flank regions of female NOD / SCID mice (7 to 8 weeks old). When the tumor reaches about 100mm 3 At the same time, the mice were randomly divided into 4 groups, 4 mice in each group, given (intravenous injection) normal saline, nant-paclitaxel, γ-irradiated (10Gy) aNK cells / HER2.taNK cells, or nant-paclitaxel plus γ-irradiated (10 Gy) aNK cells / HER2.taNK cells (γ-irradiated cells to prevent cell replication). Tumor growth was measured with calipers twice a week prior to dosing and then twice a week; animals were weighed prior to dosing before cell injection and then twice a week. All da...
Embodiment 2
[0062]In a second exemplary treatment approach to determine the efficacy of haNK cells in combination with trastuzumab in a mouse model of HER2-positive breast cancer, the inventors used haNK cells as previously described. haNK cells were developed by transfecting the parental aNK cell line with a bicistronic plasmid vector containing a high-affinity V variant of CD16 (with a valine at position 158) and intracellularly retained IL-2, which renders haNK cells Able to grow in the absence of exogenous IL-2. This plasmid contains some human sequences of CD16 and IL-2, neither of which has any transforming properties.
[0063] MDA-MB-453 cells (0.1 mL of 1×10 8 / mL in 50% Matrigel) subcutaneously into female NOD-SCID IL2Rγ 空 (NSG, Jackson Laboratory) Left and right flank regions of mice (7 to 8 weeks old). Such as Figure 4A As shown, when the tumor reaches about 100mm 3 , mice were randomly assigned to one of 10 groups of 4 mice each, and physiological saline (PBS), IgG 1 (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com