Fungicidal mixtures
A composition and compound technology, applied in the direction of fungicides, biocides, biocides, etc., can solve the problems of destructive and difficult control of plant diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] (4) Another method described in the literature for the preparation of some 2-aryl-2-hydroxy esters and acids
[0050] The method is to use activated carbonyl compound acyl in the presence of protonic acid or Lewis acid
[0051] Aromatic rings. Aromatic substrates capable of such reactions are benzene, diphenyl ether and
[0052] Other aromatics known to be sufficiently reactive to perform Friedel-Crafts type reactions
[0053] compound. In the case of monosubstituted benzene derivatives, acylation is preferentially but not necessarily exclusive
[0054] occurs at the para position to the point of attachment of the substituent. See, for example, Org.
[0055] Synthesis, Coll.Vol.3, 326, (1955), Salomon et al., Journal of Organic Chemistry
[0056] [J.Org.Chem.] (1982), 47,4692 and U.S. 4,922,010.
[0057] Carbonyl compounds known to undergo this reaction include pyruvates and acids, glyoxylates and acids, oxymalonate diesters. The acids used i...
Embodiment A
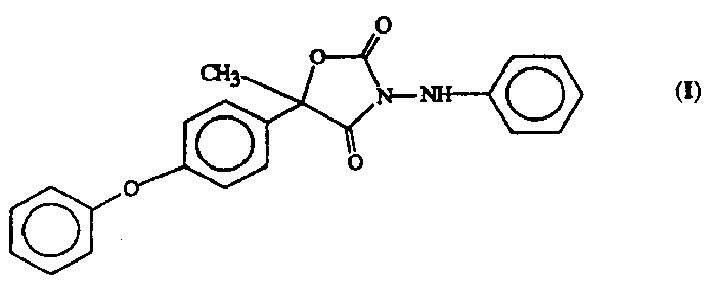
[0077] Wettable powder 5-methyl-5-(4-phenoxyphenyl)-3-phenylamino-2,4-oxazolidinedione 27.9% cymoxanil 37.1% dodecylphenol polyethylene glycol Alcohol ether 2.0% Sodium lignosulfonate 4.0% Sodium aluminosilicate 6.0% Montmorillonite (calcined) 23.0%
Embodiment B
[0079] Granules 5-methyl-5-(4-phenoxyphenyl)-3-phenylamino-2,4-oxazolidinedione 5.0% cymoxanil 5.0% attapulgite granules (low volatile material, 0.71 / 0.30mm; American sieve 25-50 mesh 90.0%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com