A novel squalene Hopaene cyclase and its application
A technology for enhopaene cyclase and cyclase, which is applied in the field of functional enzyme screening and can solve the problems such as the limitation of large-scale application by separation and purification methods.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
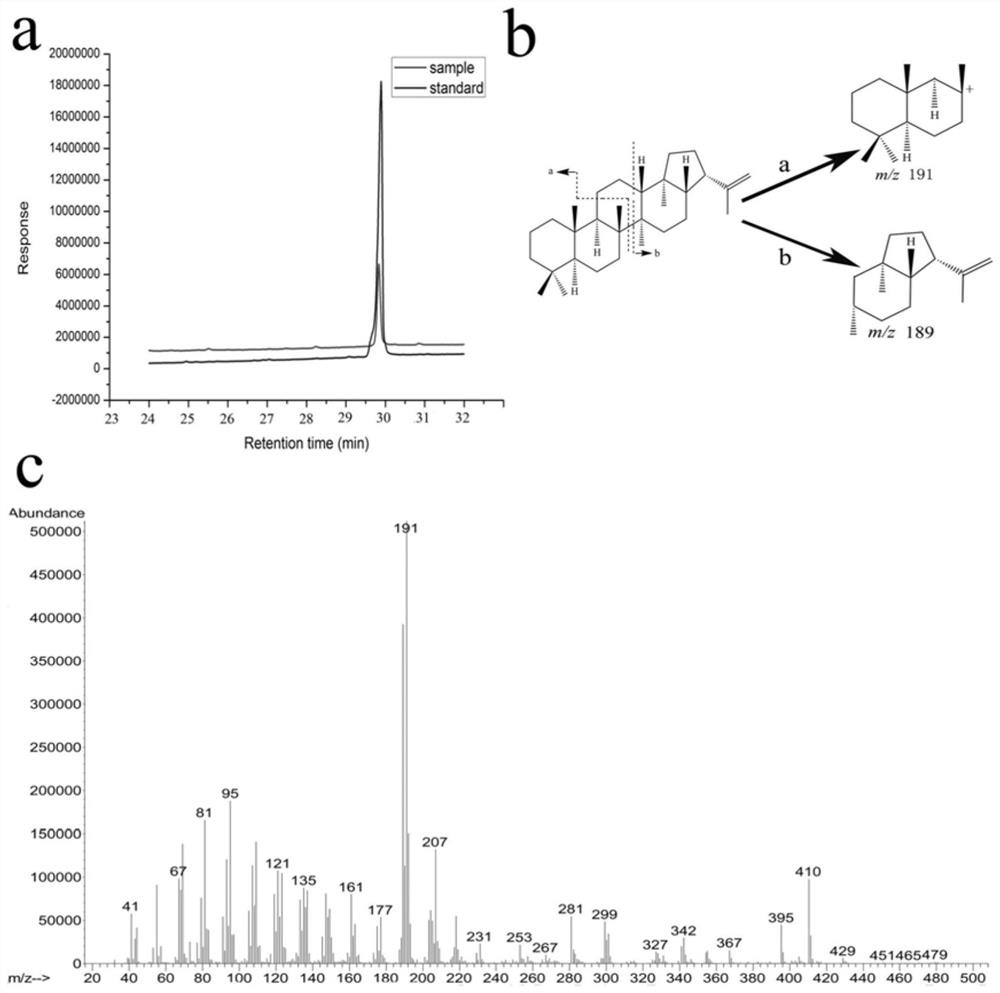
[0025] Example 1: Source and heterologous expression of novel squalene Hopaene cyclase
[0026] After the whole genome sequencing of Streptomyces albolongus ATCC 27414, the gene cluster that produces hoppene was found through cluster analysis. At the same time, the key gene shc in the synthetic pathway of hoppane was also marked after gene sequencing. The amino acid sequence of its protein is SEQ ID NO:1; the nucleotide sequence of the coding gene is SEQ ID NO:2. Afterwards, the gene was expressed heterologously to further confirm its function. The vector used pET-his(+), and the host used Escherichia coli BL21(DE3)plySs.
[0027] The above-mentioned engineered bacteria were induced to ferment. After the fermentation, 50mL of the fermentation broth was centrifuged to remove the supernatant. The bacteria were first washed with 0.9% NaCl solution, and then reconstituted with 10mL Tris-HCl buffer solution (100mM, pH 8.0). Sonication was performed under an ice bath. The supernat...
Embodiment 2
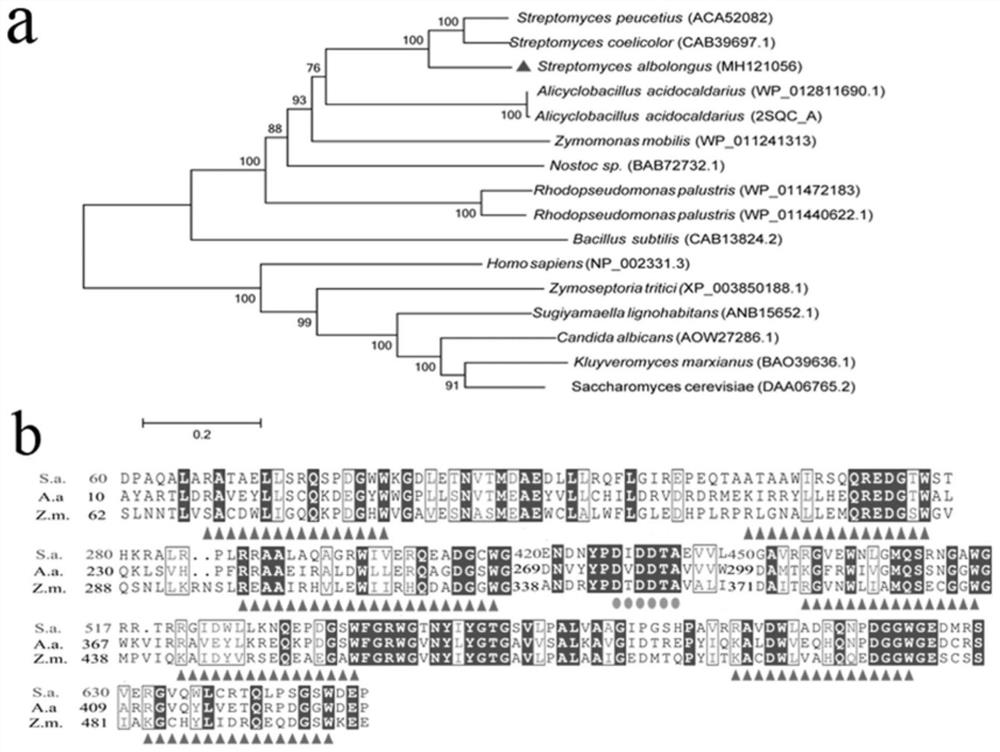
[0028] Embodiment 2: cyclase SaSHC gene sequence analysis
[0029] The family classification of cyclase SaSHC was carried out using the method reported in the literature, and MEGA 6.0 software was used to construct the evolutionary tree of cyclase SaSHC and other family cyclases, and Clustal X software was used for multiple sequence alignment of cyclases , ESPript 3.0 (http: / / espript.ibcp.fr / ESPript / ESPript / ) was used for output of aligned sequences. Such as figure 2 As shown in a, SaSHC belongs to the ISOPREN_C2_ family, and has typical cyclase conserved sequences and catalytic active sites DXDDTA and QW sequences. At the protein level, the homology of the cyclase of the present invention with the reported protein is only 70%.
Embodiment 3
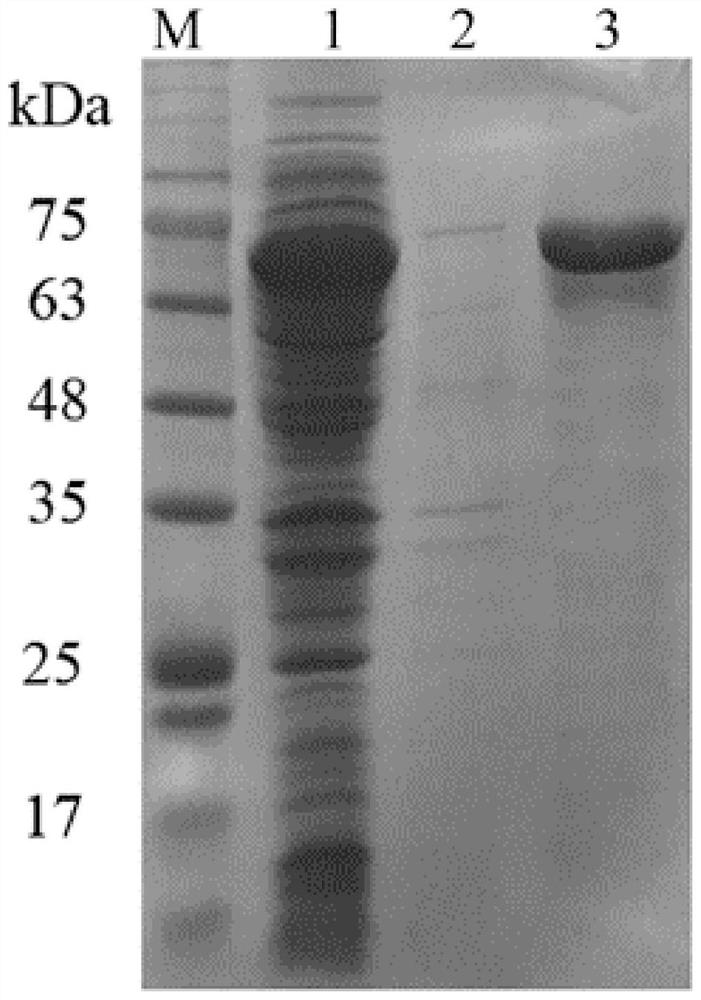
[0030] Example 3: Purification of SaSHC
[0031] SaSHC was induced to ferment again. After the fermentation, the thalline was collected by centrifugation, and the thalline was washed once with sterilized physiological saline. After washing, the cells were redissolved with 20 mM Tris-HCl buffer solution (pH 8.0), placed on an ultrasonic cell pulverizer for ultrasonic disruption, and the supernatant of the disrupted solution was used for the purification of SaSHC. The purification process was carried out using a nickel column (1 mL, Qiagen, Hilden, Germany), and the gradient elution of the protein used Tris-HCl buffer (pH 8.0, 100 mM) containing different concentrations of imidazole (20 mM-500 mM). The eluted solutions were collected separately, concentrated and desalted using an ultrafiltration concentrator tube (~30kDa), and subjected to protein electrophoresis analysis and squalene-hopene cyclase enzyme activity detection.
[0032] The protein gel electrophoresis result of a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com