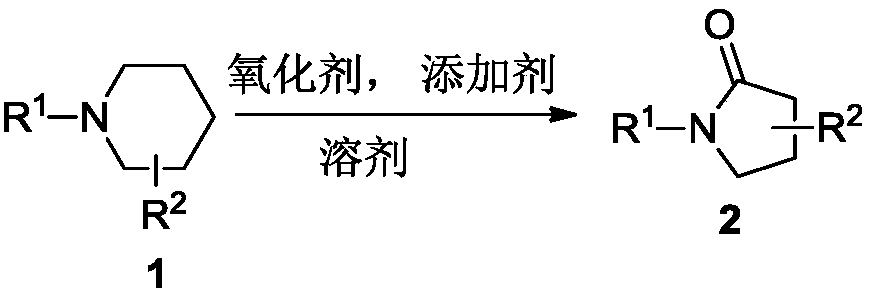
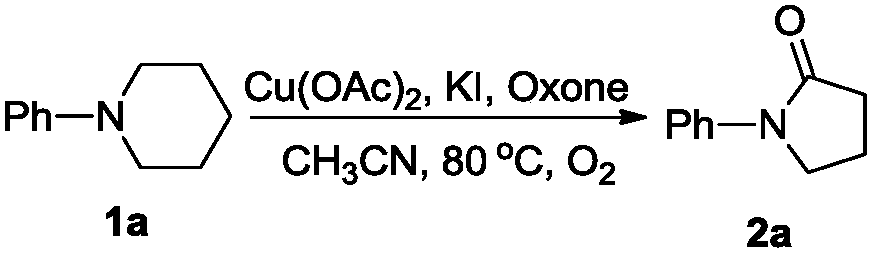Pyrrolidone compound synthesis method
A synthetic method and technology of pyrrolidone, applied in the direction of organic chemistry, can solve the problems of limited reliable methods, cumbersome operation, harsh reaction conditions, etc., and achieve the effects of wide application range, simple synthesis process, and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015]
[0016] Add 1a (0.5mmol, 81mg), acetonitrile (5mL), anhydrous copper acetate (1mmol, 181mg), potassium iodide (1mmol, 166mg) and potassium hydrogen persulfate composite salt (Oxone, 615mg, 1mmol) to a 10mL Shrek tube. ), evacuated and filled with oxygen (1atm), then placed in an oil bath at 80°C and stirred for 12h. Then, 10 mL of saturated brine was added to quench the reaction, extracted with ethyl acetate (10 mL×3), and the organic phases were combined and dried over anhydrous sodium sulfate. It was filtered, spin-dried, and separated by silica gel column (petroleum ether / ethyl acetate=3 / 1) to obtain a white solid product 2a (47 mg, 58%). The characterization data of this compound are as follows: 1 H NMR(600MHz, CDCl 3 )δ2.09(quint,J=7.8Hz,2H), 2.54(t,J=7.8Hz,2H), 3.80(t,J=7.2Hz,2H), 7.07(t,J=7.2Hz,1H) ,7.30(t,J=7.8Hz,2H),7.54(d,J=8.4Hz,2H). 13 C NMR(150MHz, CDCl 3 )δ18.1,32.8,48.8,120.0,124.5,128.8,139.4,174.2.HRMS calcd forC 10 H 11 NNaO:184.0733[M+Na] + ,found:18...
Embodiment 2
[0018] Add 1a (0.5mmol, 81mg), acetonitrile (5mL), anhydrous copper acetate (0.25mmol, 45mg), potassium iodide (0.5mmol, 83mg) and Oxone (308mg, 0.5mmol) to a 10mL Shrek tube in sequence, and vacuum After being filled with oxygen (1atm), it was placed in an oil bath at 80°C and stirred for 12 hours. Then, 10 mL of saturated brine was added to quench the reaction, extracted with ethyl acetate (10 mL×3), and the organic phases were combined and dried over anhydrous sodium sulfate. It was filtered, spin-dried, and separated by silica gel column (petroleum ether / ethyl acetate = 3 / 1) to obtain a white solid product 2a (19 mg, 23%).
Embodiment 3
[0020] Add 1a (0.5mmol, 81mg), acetonitrile (5mL), anhydrous copper acetate (0.5mmol, 91mg), potassium iodide (0.5mmol, 83mg) and Oxone (308mg, 0.5mmol) to a 10mL Shrek tube in sequence, and vacuumize After being filled with oxygen (1atm), it was placed in an oil bath at 80°C and stirred for 12 hours. Then, 10 mL of saturated brine was added to quench the reaction, extracted with ethyl acetate (10 mL×3), and the organic phases were combined and dried over anhydrous sodium sulfate. It was filtered, spin-dried, and separated by silica gel column (petroleum ether / ethyl acetate=3 / 1) to obtain a white solid product 2a (25 mg, 31%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com