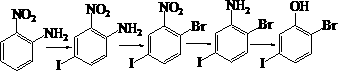
Preparation method of 2-bromo-5-iodophenol
A technology of iodophenol and iodoaniline, which is applied in the field of preparation of 2-bromo-5-iodophenol, and can solve problems such as difficult acquisition of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] (1) Iodine addition reaction: Add 100 g of o-nitroaniline and 1000 mL of solvent acetic acid into the reaction vessel. Stir, control the temperature at about 35 °C, and add 91 g of iodine in batches. After the addition, continue to stir, and GC traces until the end of the reaction. After the reaction was completed, 1000 mL of water was added, then neutralized with sodium hydroxide solution, allowed to cool, filtered with suction, and dried to obtain 180 g of light brown solid 2-nitro-4-iodoaniline, with a yield of 94%.
[0019] (2) Bromine reaction on diazo: Add 100 g of 2-nitro-4-iodoaniline to the reaction vessel, then add a solution made of 200 g of concentrated sulfuric acid and 400 mL of water, stir, and then dropwise add 37 g of A solution made of sodium nitrate and 120 mL of water was added, and the temperature was controlled at about 15 °C during the dropwise addition process. After the addition, stir for a period of time and prepare the diazonium salt for lat...
Embodiment 2
[0025] (1) Iodine addition reaction: Add 500 g of o-nitroaniline and 5200 mL of solvent acetic acid into the reaction vessel. Stir, control the temperature at about 40°C, and add 458 g of iodine in batches. After the addition, continue to stir, and GC traces until the end of the reaction. After the reaction was completed, 4000 mL of water was added, then neutralized with sodium hydroxide solution, allowed to cool, filtered with suction, and dried to obtain 930 g of 2-nitro-4-iodoaniline as a light brown solid with a yield of 97%.
[0026] (2) Bromination reaction on diazo: Add 600 g of 2-nitro-4-iodoaniline to the reaction vessel, then add a solution made of 1220 g of concentrated sulfuric acid and 2500 mL of water, stir, and then dropwise add 230 g of A solution made of sodium nitrate and 700 mL of water was added, and the temperature was controlled at about 15 °C during the dropwise addition process. After the addition, stir for a period of time and prepare the diazonium s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com